4083
Imaging plasticity associated with hippocampal kindling using simultaneous LFP-ofMRI1Department of Neurology, Stanford University, Stanford, CA, United States, 2Departments of Neurology, Bioengineering, Neurosurgery, Electrical Engineering, Stanford University, Stanford, CA, United States
Synopsis
The kindling model of epilepsy is associated with a permanent form of synaptic potentiation, which is considered to be pathological. In this study we used optogenetic fMRI to observe changes in plasticity that occur following a kindling regime. Upon stimulation of the ventral hippocampus, post-kindling fMRI data displayed significantly more widespread activation, particularly in pre-frontal regions compared to pre-kindling acquisitions. Simultaneous LFP measurements were used to confirm the absence of epileptic activity that would confound the interpretation. This represents a powerful technique which can be used for understanding cognitive impairments or understanding the mechanism of anti-epileptogenic therapies.
Purpose
The kindling model is a model of synaptic
plasticity and epilepsy. It is based upon repetitive stimulation, which leads
to a gradual increase in the susceptibility and severity of seizures. Despite
it commonly being used as a model of epilepsy, its underlying pathophysiology
is not well understood. The aim of this study was to use optogenetic functional
imaging (ofMRI) [1] with optogenetic activation of the hippocampus [2-3] to determine
how the response to stimulation of an excitatory neuronal population changes
over the whole brain after undergoing a hippocampal-kindling regime. We expected
to observe changes in synaptic plasticity in regions connected with the ventral
hippocampus. Simultaneously acquired LFP measurements were used to ensure there
were no epileptic discharges during the imaging acquisition, as these would
complicate the interpretation of the results.Methods
Surgical Procedure: Animal husbandry and experimental manipulation were in strict accordance with the NIH and Stanford University’s IACUC guidelines. Adult male Sprague-Dawley rats (n=2, kindling) and (n=1, control) were injected with CaMKIIα–ChR2(H134R)–EYFP AAV5 virus in order to drive Channelrhodopsin-2 (ChR2) expression in excitatory neurons within the ventral hippocampus. Following this, they were implanted with MRI compatible carbon fiber optrodes [3], for optogenetic stimulation and LFP readout. The animals were given more than 8 weeks to recover and to allow time for virus induced ChR2 expression.
Kindling Induction: Animals were stimulated awake and were monitored using video-LFP throughout each session. The kindling paradigm consisted of an alternate day rapid-kindling protocol. This involved 10 optogenetic stimulations every other day at 10 min intervals or until the subject experienced a Racine stage 3-5 behavioral seizure. Rats were considered kindled if the first 1-2 stimulations of the day resulted in a stage 3-5 seizure. The control subject did not undergo awake stimulation.
fMRI acquisition: fMRI imaging was performed using a 7T horizontal-bore scanner and simultaneous electrophysiology was acquired using a BrainAmp ExG Amplifier. Rats were sedated using dexmedetomidine, injected with 15 mg/kg Ferumoxytol for CBV-weighted imaging and functional imaging was implemented using a multi-slice gradient echo sequence (TR=750 ms/TE=8 ms) with a 4-interleave spiral readout. In this study we used a block-design stimulation paradigm with a sub-seizure threshold light intensity (time-averaged power density = 35-70 mW/mm2 at the fiber tip), a period of 60 s and with each period consisting of a 5 s 40 Hz pulse train (7.5 ms pulses) and a 55 s rest period.
Results
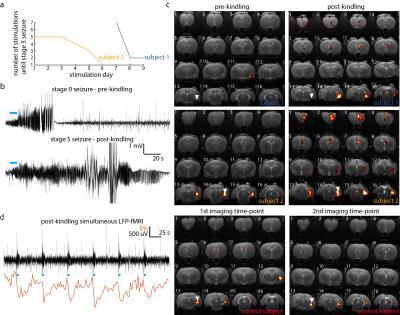
Both subjects which underwent the kindling protocol reached our definition of kindled within 9 stimulation days (Fig.1a). On the final stimulation day, both rats exhibited severe stage 5 tonic-clonic seizures, which lasted several minutes (Fig.1b-lower panel). This is in contrast to the initial pre-kindling seizures on day 0 which had no obvious behavioral manifestations (Fig.1b-upper panel). In line with our hypothesis, optogenetic stimulation of the ventral hippocampus in kindled rats leads to significantly more widespread activation compared to the initial imaging time-point or compared to both imaging time-points in the control subject (Fig. 1c). In addition to activation at the local stimulation site and septum, in both post-kindled rats, a third cluster of activity can be observed in the pre-frontal cortex. Simultaneously acquired LFP was used to confirm the absence of epileptic activity during each acquisition, which would confound the interpretation of the results (Fig. 1d).Discussion
Previous studies have used fMRI to image synaptic plasticity associated with high-frequency stimulation and long-term potentiation (LTP) [4]. In this work, we have focused on imaging plasticity associated with kindling, which while bares resemblance to LTP, is considered to be pathological and a more permanent form of synaptic plasticity. Compared to the results presented by Canals et al., who upon stimulation of the perforant path, primarily observe increased activation in the contralateral hippocampus, here, we observe changes primarily in the pre-frontal cortical regions. Furthermore, this technique of imaging kindling-induced plasticity could be used to monitor anti-epileptogenic therapies.Conclusion
We demonstrate the possibility of imaging the neuro plasticity
associated with kindling. Future work will be needed to demonstrate if these
plasticity changes persist and to disentangle the effects of LTP from effects
which are pathological.
This is a powerful technique which could be used to understand cognitive
impairments, guide the development of anti-epileptogenic therapies or as a
biomarker for monitoring treatment efficacy.Acknowledgements
This work was supported through funding from the NIH/NIBIB R00 Award (4R00EB008738), Okawa Foundation Research Grant Award, NIH Director’s New Innovator Award (1DP2OD007265), NSF CAREER Award (1056008), Alfred P. Sloan Foundation Research Fellowship, NIH/NINDS R01NS087159, NIH/NINDS R01NS091461, and NIH/NIA RF1AG047666.References
1. Lee J H, Durand R, Gradinaru V et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. 2010; Nature, 465: 788-792.
2. Weitz A J, Fang Z, Lee H J et al. Optogenetic fMRI reveals distinct, frequency-dependent networks recruited by dorsal and intermediate hippocampus stimulations. 2015; 107:229-241.
3. Duffy B A, Choy M K, Chuapoco M R et al. MRI compatible optrodes for simultaneous LFP and optogenetic fMRI investigation of seizure-like afterdischarges. 2015; Neuroimage, 123: 173-184.
4. Canals S, Beyerlein M, Merkle H et al. 2009; Current Biology, 19: 398-403.
Figures