4082
Patterns of resting-state functional connectivity in the prodromal phase of Alzheimer’s disease: insights from a tauopathy mouse model (Thy-Tau22)1ICube, Université de Strasbourg-CNRS, Strasbourg, France, 2Department of Radiology, Medical Physics, University Medical Center Freiburg, Freiburg, Germany, 3Laboratoire de Neurosciences Cognitives et Adaptatives, Université de Strasbourg-CNRS, Strasbourg, France, 4Centres Mémoire de Ressources et de Recherche, CHU de Strasbourg, Services Neurologiques et Gériatriques, Strasbourg, France, 5Département de Biophysique et Médecine Nucléaire, Hôpitaux Universitaires de Strasbourg, CHU de Hautepierre, France
Synopsis
Resting state functional magnetic resonance imaging (rsfMRI) has opened a new window into the brain and its connectome, proposing abnormal functional connectivity as a candidate biomarker of brain pathologies1. We explored this pathway and performed rsfMRI and network analysis in the Thy-Tau22 transgenic mouse model of Alzheimer’s disease, evaluating possible network signatures of early pathological states. We mapped the brain functional connectivity patterns and found overactive resting state BOLD signal in core players of the memory and learning systems, including the hippocampal network. This correlates with subtle memory deficits characterizing a very early pathological phenotype of the Thy-Tau22 mouse model.
Introduction and purpose
Neurodegenerative diseases like Alzheimer disease (AD) are a big challenge for society. One of the drawbacks in their management and treatment is the absence of clear biomarkers of the prodromal phases of the disease, leading to late diagnosis.
In this context, the main goal of our study was to identify specific patterns of resting-state functional connectivity (rsFC) that might underpin early pathological mechanisms in AD. For this, we investigated the brain functional circuitries in the Thy-Tau22 transgenic mouse model of tauopathy that develops overtime classical AD hallmarks, such as TAU protein hyperphosphorylation and its aggregation throughout the brain2,3. The rsfMRI study was therefore carried out at the onset of very subtle behavioral impairments related to learning or memory.
Methods
Two groups of 4 month wild-type (WT) C57Bl6/J mice (n=13) and Thy-Tau22 (n=13) mice were characterized. At this age, Thy-Tau22 mice don’t show any cognitive decline2 but present the first neurofibrillary tangles in hippocampus3.
Behavioral tests:
1. The hippocampus-dependent spatial learning and memory abilities were examined in the standard hidden-platform acquisition and retention version of the MWM4. 2. The two cohorts were also tested in an object exploration paradigm. This evaluates the spontaneous tendency of mice to preferentially explore new objects (object recognition) or objects moved to a new location (spatial novelty). Behavioral data were analyzed using one way analysis of the variance (ANOVA) and when appropriate with repeated measure factors (days), and one sample t-test.
MRI experiments and data analysis:
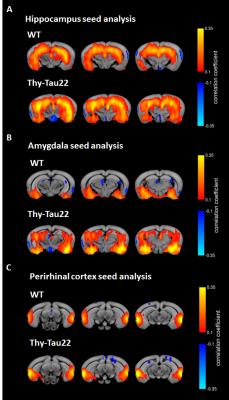
One week after behavioral evaluation, brain MRI was carried-out in the two animal groups using a 7T small bore animal scanner (BioSpec 70/30, Bruker, Germany) and a transmit/receive system composed of a volume coil (86 mm inner diameter) and a mouse head adapted, room temperature surface coil. Resting state fMRI data were acquired using single shot GE-EPI (TE/TR = 15ms/2000ms), 27 axial slices of 0.4mm thickness (FOV: 2.12x2 cm², resolution: 0.14×0.22mm2, 500 volumes). Mouse physiological conditions were monitored and stabilized during imaging performed under medetomidine (MD, Domitor, Centravet, Nancy, France) sedation (subcutaneous bolus of 0.6mg MD/kg body weight (BW) followed by s.c. infusion of 0.3mg MD/kg-BW/hour). rsfMRI data pre-processing was performed using SPM 8 for motion correction, coregistration to a study based template, spatial normalization to the Allen Mouse Brain Atlas5, a smoothing of 2 voxels and a frequency filtering from 0.01 to 0.1Hz. Regions of Interest (ROIs) were extracted from the Allen Mouse Brain atlas and used in a seed-based correlation analysis. We exemplify here the results obtained for brain areas selected in coherence with their implications in the previously described behavioral tests: hippocampus region (Ammon's horn and dentate gyrus (Fig 3A)), perirhinal cortex (Fig.3C) and amygdala (Fig.3B).
Results and Discussion
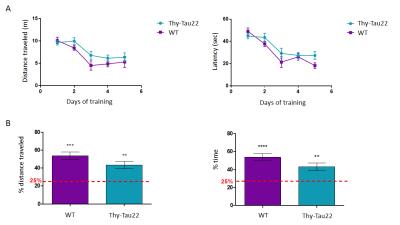
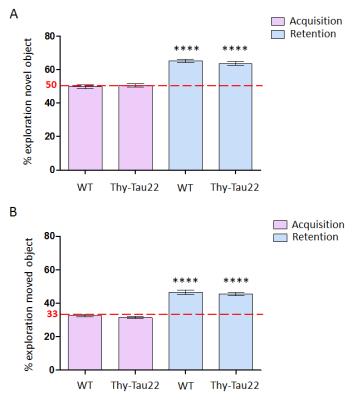
In the MWM tests Thy-Tau22 mice do not show statistically significant learning deficits compared to the controls (Fig.1.A) at 4 months of age. This time-point is considered a very early phase in the development of the pathology in this model3. Transgenic mice however display a memory decline (Fig.1.B) which could eventually be related to the formation of the first neurofibrillary tangles in the hippocampus2, which is important in this task. Furthermore, the performance of the Thy-Tau22 animals in the object recognition and spatial novelty tasks (Fig.2) (mostly related to the hippocampus, entorhinal and perirhinal cortices6) was found to be normal.
rsfMRI mapping of functional connectivity (FC) networks in Thy-Tau22 mice however showed abnormal modifications in BOLD signal for several networks, including hippocampus, amygdala and perirhinal cortices.
Interestingly, the hippocampus – amygdala FC seem to be strongly increased in the transgenic animal (Fig.3A and B). In fact, hyper-synchrony of hippocampus resting state signal was recently reported in two mouse models of amyloid plaques deposition in the early stage of the disease. The results are also consistent with the observation of hippocampal hyperactivity in mouse models of amyloidosis using two-photon Ca2+ imaging7,8. However, to our knowledge this is a new observation in tauopathy models with Thy-Tau22 mice. Moreover, perirhinal cortex seed-based analysis showed different FC pattern between WT and Thy-Tau22 mice, without showing obvious hyper-synchrony in transgenic mice (Fig.3C) revealing that increasing FC only occur in specific regions.
To conclude, these results suggest that the overactive resting state hippocampal network may be considered as a read-out and biomarker of early AD pathology, potentially underlying subtle synaptic modifications. Further analysis of the rsfMRI data should provide insight about whole brain functional network responses and adaptations in the prodromal phases of AD.
Acknowledgements
No acknowledgement found.References
1. Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13)
2. Van der Jeugd A, Vermaercke B, Derisbourg M, et al. Progressive age-related cognitive decline in tau mice. J of Alzheimer’s Dis: JAD. 2013;37:777-788.
3. Schindowski K, Bretteville A, Leroy K, et al. Alzheimer’s Disease-Like Tau Neuropathology Leads to Memory Deficits and Loss of Functional Synapses in a Novel Mutated Tau Transgenic Mouse without Any Motor Deficits. The American Journal of Pathology. 2006;169(2):599-616.
4. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of Neuroscience Methods. 1984;11(1):47-60.
5. Oh SW, Harris JA, Ng L, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508(7495):207-214.
6. Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neuroscience & Biobehavioral Reviews. 2007;31(5):673-704.
7. Busche MA, Chen X, Henning HA, et al. Critical role of soluble amyloid- for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences. 2012;109(22):8740-8745.
8. Busche MA, Eichhoff G, Adelsberger H, et al. Clusters of Hyperactive Neurons Near Amyloid Plaques in a Mouse Model of Alzhimer’s Disease. Science. 2008,321(5896)1686-1689
Figures