4079
Ultra high resolution MR histology using ROI-extraction and SNR efficient gradient echo imaging1Biochemistry, Cambridge University, Cambridge, United Kingdom, 2Translational Neuroradiology Section, National Institute of Neurologic Disorders and Stroke, Bethesda, MD, United States, 3Clinical Neuroscience, Cambridge University, Cambridge, United Kingdom
Synopsis
Custom-built solenoid coils and 3D-printed rat brain slicers were used to identify and extract surgically placed inflammatory regions of interest within rat brains. The sequentially smaller and more sensitive coils combined with a highly-optimized gradient echo sequence, imaging setup, and registration algorithm achieved ultra high 25 µm isotropic resolution 3D MRI datasets. These images contained fully sampled k-space of the tissue pathology with preservation of the high frequency image details.
Target audience
Pre-clinical scientists and translational neuroscientistsPurpose
MR histology has the advantage of direct and full 3D sampling of tissue in an inherently digital and nondestructive manner, but is limited by relatively poor resolution compared to conventional histological techniques.1 The following method was designed to increase the resolution of ex vivo tissue MRI to approach that of histology, enabling routine MR histology of rat brains on ordinary preclinical MR scanners.Method
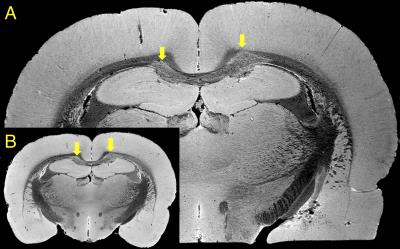
Brains surgically implanted with inflammatory lesions were extracted from rats (n=14) and fixed via submersion in warm 10% buffered formalin containing 2mM Gadovist (Bayer AG) for 3-5 days. Brains were then submerged in a fluoropolymer (Fomblin Solvay S.A) and imaged with a Varian 9.4T scanner with a 400mT/m 205/120/HD gradient insert (Magnex Scientific Ltd). Images were obtained with a 38 µm isotropic resolution (n=14) overnight scan (9-16 hours) in a 24 mm 4-turn solenoid coil (Fig. 1A), a brain slicer was 3D-printed and used to extract the lesioned area (Fig 1C), which was then scanned at 25µm isotropic resolution (n=7) over a full day (20-30 hours) in a 15 mm 6-turn solenoid coil (Fig. 1B).
A gradient-spoiled gradient echo sequence was designed to maximize SNR and resolution using an approach that: 1. minimized gradient duty cycle while reducing TR and 2. minimized receiver bandwidth while reducing TE. Gradient duty cycle was minimized by orienting tissue at 45° to each of the three gradients, reducing the duty cycle for individual gradients in each step of the pulse sequence. TE was minimized by using a short, 70µs Gaussian excitation pulse with broad, 16x slab thickness to maintain B₁ homogeneity and by using fast, high current phase-encoding steps with gradient strength up to 16 G/cm. To maintain tissue contrast and increase SNR while reducing duty cycle, receiver bandwidth was lowered to 67 Hz/px (TE≅10ms) for 38µm isotropic images and 58 Hz/px (TE≅12.5 ms) for 25µm isotropic images. Flip angle was set to the Ernst angle (40-50°) for each sample. To account for tissue heating and drift, multiple acquisitions were taken and registered to a central time point before averaging.
Results and Discussion
Custom-built solenoid coils enabled optimal filling factor and a substantial increase in SNR compared to other coil types. The 38µm isotropic MRI (Fig. 2B) was used for full tissue sampling to determine the extent of abnormal pathology and to build a 3D printed brain slicer for the tissue to extract the affected region.2 This allowed the tissue of interest to fit in a smaller 15 mm solenoid coil with double the SNR and enabling a higher resolution at 25 µm isotropic (Fig. 2A) with an optimized gradient echo sequence.
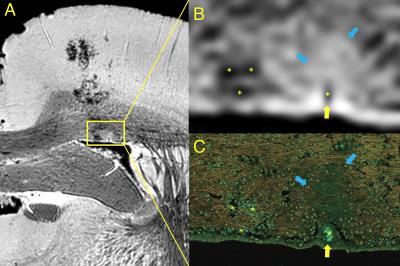
Rotation of the tissue 45° to each gradient axis enabled shortening of the TR from 35 to 20 ms for the 38 µm isotropic images and from 50 to 30 ms for the 25 µm isotropic images without increasing the duty cycle of the gradient hardware. This proportionally reduced scan time and effects from motion and frequency drift caused by tissue heating. Reduction of the TE by using a quick 70µs Gaussian pulse with large slab thickness further decreased the slice selection duty cycle. The reduction of receiver bandwidth reduced the readout gradient duty cycle while increasing SNR and tissue contrast without chemical shift artifacts or significant distortion due to the single tissue type present within a fluorinated medium. Registration between acquisitions of the tissue was necessary to maintain the high frequency details, which was done using a 9 degrees of freedom algorithm registering images to a central time point. The flat surface of the extracted tissue resulted in the same orientation of MR images to histological sections (Fig 3). Immersion-fixation of the tissue in warm gadolinium-laden formalin used in the method simplified the procedure to shorten tissue T₁ homogeneously and enabled high-resolution retrospective imaging on already-obtained tissues.
Conclusions
The combination of custom-built solenoid coils, extraction of the imaging region of interest, and use of an optimized gradient echo sequence enabled routine MR histology of rat brains with resolutions approaching the level of conventional histology. Unlike histology, MR histology is fully 3D, non-destructive, inherently digital, has no direct cost, and directly measures tissue properties that can be correlated directly to other MR-based techniques. The protocol used in this study used overnight and weekend scanner time that did not interrupt regular scanning hours.Acknowledgements
No acknowledgement found.References
1. Johnson, G. A., Ali-Sharief, A., Badea, A., Brandenburg, J., Cofer, G., Fubara, B., ... & Upchurch, L. (2007). High-throughput morphologic phenotyping of the mouse brain with magnetic resonance histology. Neuroimage, 37(1), 82-89.
2. Guy, J. R., Sati, P., Leibovitch, E., Jacobson, S., Silva, A. C., & Reich, D. S. (2016). Custom fit 3D-printed brain holders for comparison of histology with MRI in marmosets. Journal of Neuroscience Methods, 257, 55-63.
Figures