4078
Effects of taurine on resting-state fMRI activity in rat model of attention deficit hyperactivity disorder1Department of Medical Imaging and Radiological Sciences, Chung Shan Medical University, Taichung, Taiwan, 2Institute of Biochemistry, Microbiology and Immunology, Chung Shan Medical University, Taichung, Taiwan, 3School of Medicine, Chang Gung University, Taoyuan, Taiwan, 4Department of Psychiatry, Chang Gung Memorial Hospital, Chiayi, Taiwan, 5Department of Medical Imaging, Chung Shan Medical University Hospital, Taichung, Taiwan
Synopsis
Attention deficit hyperactivity disorder (ADHD) is a most common developmental disorders in both children and adult population. However, the treatment for ADHD remains limited. To investigate the effects of taurine on ADHD, a spontaneously hypertensive rat (SHR) animal model was adopted in this study. The functional brain signals including functional connectivity (FC) and mean amplitude of low-frequency fluctuation (mALFF) were detected by using resting-state functional magnetic resonance imaging (rs-fMRI). Our findings in FC and mALFF suggested that taurine administration probably improves the hyperactive behavior in ADHD by changing brain functional signals in SHR rats.
Introduction
Attention deficit hyperactivity disorder (ADHD) is a most common developmental disorders in both children and adult population, with a prevalence of 5-10% 1 and 3-5% 2, respectively. Taurine, known as the most abundant amino acids in the central nervous system, is used by the body for a variety of functions 3,4. It has been reported strongly associated with various ADHD-related neurotransmitters 5. Therefore, this study intends to investigate the effects of taurine on ADHD by brain functional activity in spontaneously hypertensive rats (SHR), an animal model of ADHD.Materials and Methods
As a valid and accepted animal model for ADHD, three-week-old male spontaneously hypertensive rats (SHR, N=22) (body weight: approximately 180g) and its relative control, Wistar-Kyoto (WKY, N=25) rat, were adopted in this study 6,7. Two kinds of rats were separately divided into control, low taurine (22.5 mmol/kg taurine) and high taurine (45 mmol/kg taurine) group. Whole brain resting- state functional images were acquired from all subgroups mentioned above and scanned by 7T MRI (Bruker BioSpin, Ettlingen, Germany) by echo planar imaging (EPI) with the following parameters: repetition time/echo time (TR/TE) =2000ms /20 ms, resolution (voxel size) = 3.12 x 3.12 x 1 mm3, slice number = 12, number of repetition = 300, and the scan time = 10 min. The raw data were first converted into ANALYZE format. The preprocess were then performed, including slice timing, realignment, denoise, detrending and filtering (0.01-0.12 Hz), using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK)and Resting-State fMRI Data Analysis Toolkit (REST1.8, Lab of Cognitive Neuroscience and Learning, Beijing Normal University, China) . The functional connectivity (FC) and mean amplitude of low-frequency fluctuation (mALFF) were then calculated by using REST1.8.Results
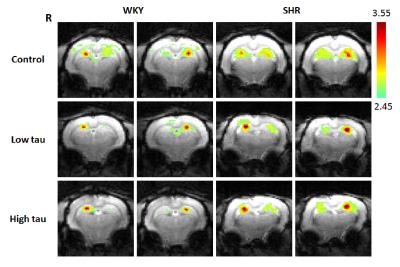
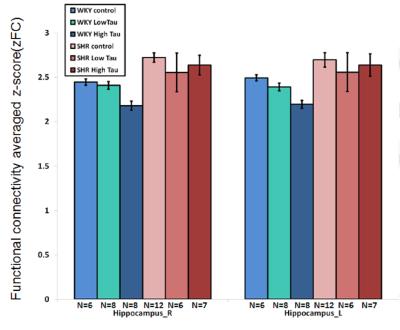
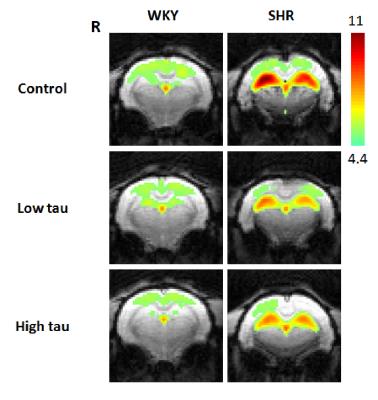
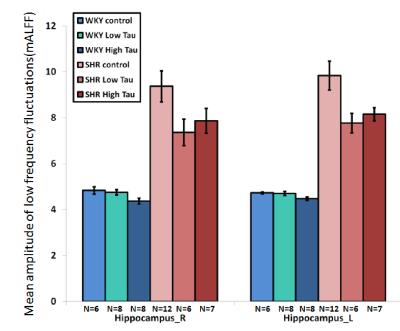
In Fig. 1 and 2, significantly higher FC of the bilateral hippocampus in rats from SHR control group was detected as compared to those from WKY control group. After low or high-dose taurine treatment, the FC of the bilateral hippocampus in SHR rats returned to the levels in rats from WKY control group. Significantly decreased FC of the bilateral hippocampus was observed in rats from WKY high-taurine group as compared to those from WKY control group (Fig. 2). In Fig. 3 and 4, the mALFF of the bilateral hippocampus in rats from SHR control group was significantly higher as compared to those from WKY control group. Significantly decreased mALFF of the bilateral hippocampus in rats from WKY high-taurine group was observed as compared to those from WKY control group (Fig. 4). After low- or high-dose taurine treatment, the mALFF of the bilateral hippocampus in rats from SHR low-taurine and high-taurine groups significantly decreased as compared to those from SHR control group, respectively.Discussion
Evidence has indicated that hippocampal enlargement may represent neural responses within the hippocampus that compensate for problems in temporal processing and delay aversion in ADHD 8. However, these findings are not very stable across samples from different ADHD patients and that the different and contradictory findings may be related to the different locations of alterations along the complex circuits responsible for the different symptoms of ADHD 9,10.A previous study reported that ADHD patients exhibited higher ALFF values in the left superior frontal gyrus and sensorimotor cortex (SMC) and suggested abnormal frontal activities at resting state associated with underlying physiopathology of ADHD 11. Other evidence also indicated that ADHD patients exhibited impaired executive function, along with the abnormal fMRI indices such as higher ALFF in the left globus pallidus, the right globus pallidus and the right dorsal superior frontal gyrus, and higher FC in the frontostriatal circuit relative to the healthy controls 12. Although the status of brain fMRI signals and altered brain connectivity in the large-scale resting-state networks remain conflicting in ADHD 12, this study revealed beneficial effects of taurine on improving the ADHD-like behavior in SHR rats. Anyway, further investigations are required to clarify the precise associations between fMRI indices and ADHD symptoms.Conclusion
This study for the first time revealed the beneficial effects of taurine on an ADHD animal model. After low or high-dose taurine treatment, the FC of the bilateral hippocampus in SHR rats returned to the levels in rats from WKY control group. Additionally, taurine significantly reduces the mALFF of the bilateral hippocampus in SHR rats. These preclinical findings indicate that taurine may present an alternative remedy for ADHD treatment. However, further animal and clinical studies are required in order to examine the precise mechanism, efficacy, safety and optimal dosage prior to offering taurine in clinical application.Acknowledgements
This work was supported in part by Chang Gung Medical Foundation, Chiayi Chang Gung Memorial Hospital and Chang Gung University, Taiwan, and Center of Excellence for Clinical Trial and Research, Ministry of Health and Welfare, Taiwan (CMRPG6E0261).References
1. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–48.
2. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–723.
3. Lima L, Obregon F, Cubillos S, et al. Taurine as a micronutrient in development and regeneration of the central nervous system. Nutr Neurosci. 2001;4:439–443.
4. Bidri M, Choay P. Taurine: a particular amino acid with multiple functions [in French]. Ann Pharm Fr 2003;61:385–391.
5. El Idrissi A, Boukarrou L, Dokin C, et al. Taurine improves congestive functions in a mouse model of fragile X syndrome. Adv Exp Med Biol. 2009;643:191–198.
6. Ueno K, Togashi H, Matsumoto M, et al. Alpha4beta2 nicotinic acetylcholine receptor activation ameliorates impairment of spontaneous alternation behavior in stroke-prone spontaneously hypertensive rats, an animal model of attention deficit hyperactivity disorder. J. Pharmacol. Exp. Ther. 2002;302(1):95–100.
7. Sagvolden T, Russell VA, Aase H, et al. Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1239–47.
8. Frodl T, O'Keane V.How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis. 2012;52:24–37.
9. Plessen KJ, Bansal R, Zhu H, et al. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:795–807.
10. Perlov E, Philipsen A, Tebartz van Elst L, et al. Hippocampus and amygdala morphology in adults with attention-deficit hyperactivity disorder. J Psychiatry Neurosci. 2008;33:509–15.
11. Yang H, Wu QZ, Guo LT, et al. Abnormal spontaneous brain activity in medication-naïve ADHD children: A resting state fMRI study. Neuroscience Letters. 2011;502:89–93.
12. Li F, He N, Li Y, et al. Intrinsic brain abnormalities in attention deficit hyperactivity disorder: a resting-state functional mr imaging study. Radiology. 2014;272(2):514–23.
Figures