4077
Myelin degeneration in cynomolgus macaques with advanced age using quantitative myelin imaging1Institute of Neuroscience, Chinese Academy of Sciences, Shanghai, People's Republic of China, 2Center for Brain Imaging Science and Technology, Department of Biomedical Engineering, Zhejiang University, Hangzhou, People's Republic of China
Synopsis
Effects of normal aging on myelin have drawn considerable attention for decades. Previous studies hypothesized that myelin sheath degeneration could result in the reduction of the fraction of myelin water due to the inflow of intra- or extra-cellular water between myelin sheath. This study investigates aging effects on myelin degeneration in elderly macaques using a multi-echo GRE sequence. Using a three-pool model, we observed a significant decrease in the fraction of myelin water in the body of corpus callosum and a negative correlation with ages in 8 monkeys (aged 21~25), indicating heterogeneous degradation of white matter infrastructure with aging.
Introduction
Effects of normal aging on myelin have drawn considerable attention for decades. Histological studies in macaques have shown that two main types of myelin sheath degeneration occur with aging: formation of myelin balloon, and accumulation of dark cytoplasm from oligodendrocytes or Schwann cells. It has been suggested the myelin sheath splits and stretches to make room for the myelin balloon or dilated cytoplasm, which contains mostly extracellular or intracellular water, respectively1. In both kinds of myelin degeneration, the fraction of myelin water (defined as the amount of bounded water between membranes characterized by a short T1, T2 and T2*) reduces significantly due to the inflow of intracellular or extracellular water between myelin sheath. Although MRI techniques has demonstrate great potentials to noninvasively detect demyelination, to the best of our knowledge, no quantitative studies of myelin degeneration with aging have been done on nonhuman primates in vivo. This study aims to examine aging effects of myelin degeneration in elderly macaques using a multi-echo GRE (mGRE) sequence.Methods
Eight elderly macaques (age 23.1±1.0, weight 6.4±2.2 kg, 6 male) were scanned on a 3T Siemens Trio scanner with AC88 gradient-insert. A 3D mGRE sequence was launched for data acquisition: TR 60ms, 32 echoes, TE of 1st echo 2.4ms, echo spacing 1.42ms, matrix size 128×128×52, 1mm isotropic voxel size, flip angle 25 deg, 6 averages. T1 weighted MPRAGE images were also acquired. The acquired mGRE images were filtered using anisotropic diffusion filter to enhance SNR. These voxel-wise T2* decays were fitted using a three-pool model2 :
$$S(t)=A_{my}e^{-(1/T_{2,my}^*)} +A_{ax}e^{-(1/T_{2,ax}^*)} +A_{ex}e^{-(1/T_{2,ex}^*)} $$
where A represents signal magnitude of each water component. Subscripts my, ax, and ex denote myelin water, axonal water and extracellular water, respectively. Myelin water fraction (MWF) mapping was calculated voxel-by-voxel as MWF = Amy / (Amy + Aax + Aex)3. The resultant MWF map was then co-registered with the INIA19 template. Correlation analysis was performed to investigate MWF in eight ROIs co-variation with age. These ROIs were bilateral body, splenium, genu of corpus callosum (CC) and bilateral internal capsule.
Results
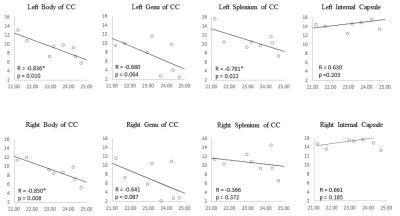
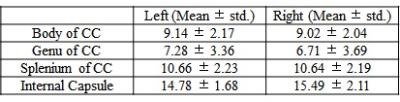
MWF values of these 8 ROIs are shown in Table 1 (in unit of percentage). Fig. 1 shows sagittal MWF maps for one 21 and one 25 years old monkeys overlaid on the INIA19 template. Red and orange dashed lines indicate the spatial location of the body of CC in the left and right hemispheres, respectively. In these regions, the MWFs are evidently much lower in the 25-year-old monkey. Fig. 2 plots the correlation results of MWF with age in the selected ROIs for all eight macaques. The MWFs in the internal capsule did not show a clear correlation with ages (p>0.1). The MWFs in other six ROIs demonstrated a decreasing tendency with ages, where left splenium and bilateral body of CC were statistically significant (p<0.05).Discussion and Conclusion
Table 1 presents that MWF values vary among the selected ROIs, whereas bilateral similarity of those MWFs demonstrates the stability of the calculated MWF maps. Histological studies imply an increment of free water during myelin degeneration and thus a reduction of signal fraction in bounded myelin water, for both myelin balloons and cytoplasm dilation1,4,5.We found that MWF exhibited a tendency to decrease with ages in six out of eight ROIs, and three of them showed significant (p < 0.05) inverse relation. In short, we observed decreased MWF of most ROIs in elderly macaques and a significant negative correlation with their ages, substantiating heterogeneous aging-dependent myelin degradation. Future investigations should include a group of younger samples to explore the relationship between myelin degradation and aging throughout the lifespan.Acknowledgements
No acknowledgement found.References
1. Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol 2002;31(8-9):581-593.
2. Lancaster JL, Andrews T, Hardies LJ, Dodd S, Fox PT. Three-pool model of white matter. J Magn Reson Imaging 2003;17(1):1-10.
3. Hwang D, Kim DH, Du YP. In vivo multi-slice mapping of myelin water content using T2* decay. Neuroimage 2010;52(1):198-204.
4. Feldman ML, Peters A. Ballooning of myelin sheaths in normally aged macaques. J Neurocytol 1998;27(8):605-614.
5. Peters A. The effects of normal aging on myelinated nerve fibers in monkey central nervous system. Front Neuroanat 2009;3:11.
Figures
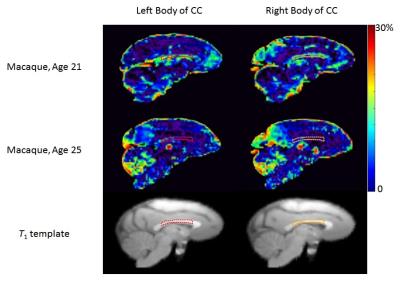
Figure 1: MWF map (Sagittal view) for one 21 and one 25 years old macaque monkeys. Red and orange dash lines indicate the body of corpus callosum (CC) in the left and right hemispheres, respectively. The T1 template shows the position of the defined ROIs.