4075
Diffusion MRI Quantifies Hippocampal CA1 Dendritic Loss and Inflammation in TMEV-Induced Epilepsy1Radiology, The First Affiliated Hospital of Nanchang University, Nanchang, People's Republic of China, 2Radiology, Washington University in St. Louis, St. Louis, MO, United States, 3Pathology, University of Utah, Salt Lake City, UT, United States, 4Chemsitry, Washington University in St. Louis, St. Louis, MO, United States, 5Biomedical Engineering, Washington University in St. Louis, St. Louis, MO, United States, 6Biostatistics, Washington University in St. Louis, 7Hope Center for Neurological Disorders, Washington University in St. Louis, St. Louis, MO, United States
Synopsis
Hippocampal neuronal damage and inflammation are the hallmark pathologies of epilepsy. We demonstrate the capability of diffusion based spectrum imaging (DBSI) to quantify hippocampal CA1 neuronal dendritic injury/loss and inflammation in TMEV-induced epilepsy mice, followed by immunohistochemistry (IHC) validation. Results demonstrate that both DTI and DBSI metrics changed in CA1 region of TMEV-induced seizure mouse. DBSI-derived fiber fraction correlated with MAP2-positive area fraction, and DBSI-derived restricted isotropic diffusion fraction correlated with DAPI-positive nucleus density.
Purpose
Although the mechanisms underlying epilepsy vary with the type of inciting events and are often multifactorial, inflammation cascade seems to be a common underlying factor for seizures 1,2. Hippocampus is particularly vulnerable to epilepsy and is highly associated with inflammation 3. A quantification of inflammation together with tissue injury in hippocampus could accurately reflect the underlying pathologies and disease progression in epilepsy. In this study, we used novel diffusion based spectrum imaging (DBSI) to detect and quantify hippocampal CA1 neuronal dendritic injury/loss and concomitant inflammation in TMEV-induced epilepsy mice.Materials and Methods
Animal model: Four- to five-week old male C57BL/6 mice were injected with 20 µl of either phosphate buffered saline (PBS) or 3×104 plaque forming units of Daniels (DA) strain of TMEV intracerebrally under isoflurane anesthesia, as previously described 4. Mice were observed continuously for 2 hours/day for 6 days to monitor seizure activity. TMEV-infected mice that had acute behavioral seizures (n=5) and PBS-infected mice (n=5) were perfusion fixed on day 6 after infection. DBSI: A surface coil (17 mm in diameter and 27 mm in length) was used to cover the whole brain. DBSI was performed on an 11.74-T Agilent small-animal MR scanner utilizing a multiple-echo spin-echo diffusion-weighted sequence 5. A 99-direction diffusion scheme was performed. All images were obtained with following acquisition parameters: TR = 3,000 ms, TE = 32.8 ms, Δ = 18 ms, δ = 5 ms, FOV (field-of-view) = 19.2 × 19.2 mm2, matrix size = 192 × 192, slice thickness = 0.5 mm, gap = 0. The max b-value = 3030.3 s/mm2. Data analysis: A lab-developed DBSI code was performed on diffusion weighted MR data to estimate λll, λ⊥ and FA derived by DBSI and DTI, and DBSI specific restricted (putative cellularity) and non-restricted isotropic (putative edema) diffusion tensor fractions 6,7. Histology: Mice brains were embedded and sectioned after ex vivo diffusion weighted MR measurements for immunohistochemical (IHC) staining of NeuN, MAP2, IBA1 and DAPI.Results
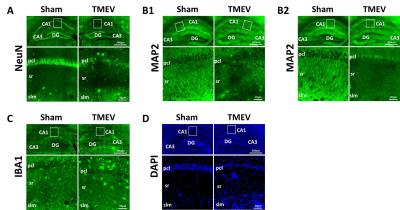
Both DTI and DBSI changed in CA1 region manifested as decreased FA (DTI: p < 0.005; DBSI p < 0.05), decreased λll (DTI, p < 0.005; DBSI, p < 0.005), decreased λ⊥ (DTI, without statistical significance; DBSI, p < 0.05), decreased DBSI-fiber fraction (p < 0.005), and increased DBSI-restricted isotropic diffusion fraction (p < 0.005) in TMEV mice (Fig. 1, and 2). IHC of neuronal death (Fig. 3A), dendrites injury/loss (Fig. 3B), activated microglia (Fig. 3C) and inflammatory cell infiltration (Fig. 3D) was observed in TMEV mouse hippocampal CA1 region. Quantitative IHC revealed significant dendritic loss (Fig. 4A) and increased cellularity (Fig. 4B) in TMEV mouse CA1. DBSI-derived fiber fraction correlated with MAP2-positive area fraction (Fig. 4C, r = 0.79, p = 0.0067), and DBSI-derived restricted isotropic diffusion fraction correlated with DAPI-positive nucleus density (Fig. 4D; r = 0.81, p = 0.0045).Discussion
Hippocampal CA1 dendritic injury/loss and inflammation was confirmed by IHC. DBSI-derived fiber fraction and restricted isotropic diffusion fraction accurately reflected dendritic density and cellularity, supported by the strong correlation with MAP2-positive area fraction and DAPI-positive nucleus density, respectively. Although not substantiated by specific staining, the extent of DTI- and DBSI-derived λll decrease in TMEV mice may reflect the severity of dendritic injury.Acknowledgements
This study was supported in part by the grants from National Institute of Health [R01-NS047592, R01-NS065714-01A1, P01-NS059560, U01-EY025500, R01-NS065714], National Multiple Sclerosis Society (NMSS) [RG 4549A4/1], and Department of Defense Idea Award [W81XWH-12-1-0457]. JZ was supported by the scholarship from Nanchang University, China.References
1. Kleen, J. K. & Holmes, G. L. Brain inflammation initiates seizures. Nat Med 14, 1309-1310, doi:10.1038/nm1208-1309 (2008).
2. Vezzani, A. Epilepsy and inflammation in the brain: overview and pathophysiology. Epilepsy Curr 14, 3-7, doi:10.5698/1535-7511-14.s2.3 (2014).
3. Vezzani, A., French, J., Bartfai, T. & Baram, T. Z. The role of inflammation in epilepsy. Nat Rev Neurol 7, 31-40, doi:10.1038/nrneurol.2010.178 (2011).
4. Libbey, J. E. et al. Seizures following picornavirus infection. Epilepsia 49, 1066-1074, doi:10.1111/j.1528-1167.2008.01535.x (2008).
5. Tu, T. W. et al. Phase-aligned multiple spin-echo averaging: a simple way to improve signal-to-noise ratio of in vivo mouse spinal cord diffusion tensor image. Magn Reson Imaging 32, 1335-1343, doi:10.1016/j.mri.2014.07.004 (2014).
6. Wang, Y. et al. Quantification of increased cellularity during inflammatory demyelination. Brain 134, 3590-3601, doi:10.1093/brain/awr307 (2011).
7. Wang, Y. et al. Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. Brain 138, 1223-1238, doi:10.1093/brain/awv046 (2015).
Figures
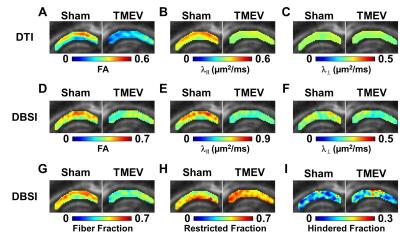
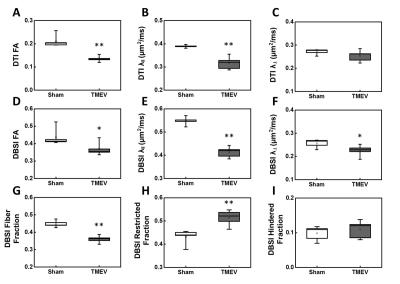
Group-averaged DTI-derived FA, λǁ and λ⊥ (A-C), and DBSI-derived FA, λǁ, λ⊥, fiber fraction, restricted isotropic diffusion fraction, and hindered isotropic diffusion fraction (D-I) were compared for CA1 between sham and TMEV mice. Decreased DTI-derived FA (A), λǁ (B) and λ⊥ (C, without statistical significance) were observed in TMEV mice. Significantly decreased DBSI-derived FA (D), λ‖ (E) and λ⊥ (F) were seen in TMEV mice. TMEV mice showed significantly decreased fiber fraction (G) and increased restricted isotropic diffusion fraction (H) compared to those seen in sham mice. *p < 0.05; **p < 0.005.
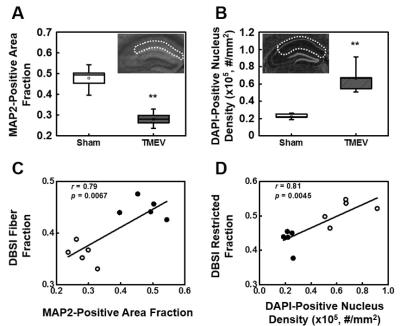
MAP2 and DAPI immunohistochemical staining was quantified in the regions outlined with dotted lines in the inset of panels A and B. MAP2-positive area fraction significantly decreased in the TMEV infected CA1 comparing with that of the sham (A). In contrast, DAPI-positive nucleus density significantly increased in TMEV infected CA1 (B). A regression analysis of MAP2-positive area fraction with DBSI-fiber fraction (C), and DAPI-positive nucleus density with DBSI-restricted isotropic diffusion fraction (D) exhibits a linear relationship between DBSI assessed dendritic density and cellularity and immunohistochemical staining results. Filled circles = TMEV; open circles = Sham; ** p < 0.005.