4072
Resting-state functional connectivity reveals deep brain stimulation and 5-HT treated alteration in autism rat1Department of Biomedical Engineering, National Yang-Ming University, Taipei, Taiwan, 2Department and Institute of Physiology, National Yang-Ming University, Taipei, Taiwan, 3Brain Research Center, National Yang-Ming University, Taipei, Taiwan, 4The PhD Program for Neural Regenerative Medicine, Taipei Medical University, Taipei, Taiwan, 5Interdisciplinary Institute of Neuroscience and Technology, Zhejiang University, Hangzhou City, Zhejiang Province, People's Republic of China
Synopsis
This study demonstrates changes of functional connectivity in motor related brain areas and the motor cortex and striatum may be crucial areas for treatment and evolution of autism spectrum disorder (ASD). Our results indicate that both CT-DBS and 5-TH treatments can alter the social interaction and motor related functional connectivity VPA-induced ASD rats by modifying the motor cortico-striatal circuit. The rsfMRI has the potential to explore functional connectivity in the brain and monitor functional plasticity changes in a specific neuroanatomical pathway in vivo.
INTRODUCTION
Autism spectrum disorder (ASD) is a lifelong neurodevelopmental disorder characterized by weak social interaction, communication, stereotyped and repetitive behaviors1. Valproic acid (VPA) can alter mood stability by modifying gamma-aminobutyric acid (GABA) levels2,3 and increase the risk of ASD4. The VPA-induced ASD model can produce genetic and behavioral phenotype with similar clinical significance of ASD5. A previous study indicated that VPA can inhibit connectivity of motor circuits in primary motor (M1) → pre-motor cortex (PMd) and M1 → supplementary area motor (SMA)6. Therefore, our hypothesis is that modulating the motor circuits may improve the social and cognitive deficits in ASD. Deep brain stimulation in the central thalamus (CT-DBS) has been demonstrated that can increase the exploratory motor behaviors and enhance cognitive performance and skill learning by increasing the cortico-striatal connectivity7,8. In addition, 5-hydroxytryptamine (5-HT) has been considered a neurochemical biomarker of ASD9. The 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT), which is a 5-HT1A receptor agonist, can increase social interaction in the VPA-induced ASD by promoting combination with 5-HT and 5-HT1A receptor10. In this study, we proposed that use of CT-DBS or 8-OH-DPAT therapies treat the VPA-induced ASD rats. Then, we used resting-state functional MRI (rsfMRI) to evaluate the changes of functional connectivity at cortico-cortical and cortico-striatal pathway for effectiveness comparison of CT-DBS and 8-OH-DPAT.METHODS
Adult female Sprage Dawley rats (weight 250-300 g) were housed in the animal facility under 12:12-h light/dark cycle (lights on at 7:00 am) with controlling temperature at 22 ± 2°C and then the rats were mated. The pregnant rats in VPA group were received a single intraperitoneal injection of VPA (500 mg/Kg) at the pregnancy day of 12-1311 and the rats in control group were injected with saline. The offspring were evaluated by a standard social interaction behavioral testing. Twenty offspring were used in this study, including the control (n=5), VPA-induced ASD (n=5), CT-DBS (n=5) and 8-OH-DPAT (n=5) groups. The social testing included three chambered facility including empty, central, and social, and how long the rats spent in each facility were calculated (Fig. 1A). In the CT-DBS group, MRI-compatible 16-channel neural probes (Fig. 1B) were stereotactically implanted into central lateral thalamus (AP: -2.5 mm, ML: ±1.4 mm, and DV: 4.5 mm) in the VPA-induced ASD rats. Then the DBS was implemented with a bipolar square-wave current of 0.4 mA with 25 μs pulse-width at 100 Hz for 30 min, once a day for three days. In the 8-OH-DPAT group, 8-OH-DPAT dissolving in 0.9% normal saline was injected (0.5 mg/d.kg body weight) intraperitoneally daily into the VPA-induced rats for 7 days. For fMRI experiments, rats were anesthetized with 0.1 mg/kg Dexdomitor® subcutaneously. MRI was performed on a Bruker Biospec 7T system with a 30-cm diameter bore and a single-shot GE-EPI sequence (TR/TE=2000/20 ms, BW=200 kHz, 80×80 matrix, FOV=25×25 mm2, thickness=1 mm, slice number = 10) was used to acquire rsfMRI images totaling 260 scanning images for 10 dummy scanning and 250 images. Functional connectivity were calculated by using Resting State fMRI Data Analysis Toolkit (REST) v1.7 with seed-based method within a 2 x 2 pixel region of interest (ROI) in left and right primary motor (M1L and M1R) and in left and right striatum (StrL and StrR). Pattern comparison of functional connectivity was computed by one sample t-test and alphasim correction and the variables of functional connectivity were assessed by Student’s t-test. The significant difference between groups was considered if p value < 0.05.RESULTS & DISCUSSION
The social interaction behavioral testing involving an unfamiliar rat chamber (social chamber) and empty region and performed using control-offspring, VPA-exposed offspring, VPA-exposed treatment with CT-DBS and VPA-exposed treatment with 8-OH-DPAT. The results revealed that the VPA-exposed offspring spent slightly less time with unfamiliar rat (social chamber) and more time in empty chamber than did the control group. Both CT-DBS and 8-OH-DPAT treatments could improve the social deficits in rats (Fig 2A). Functional connectivity among the right hemispheric motor (M1R), left hemispheric motor (M1L), right hemispheric striatum (StrR) and left hemispheric striatum (StrL) were analyzed by rsfMRI. The connectivity between M1L–M1R, M1L–StrL and M1R–StrR was decreased in VPA-induced ASD group. The effectives treatment of CT-DBS and 8-OH-DPAT could improve the functional connectivity of M1L–StrL and M1R–StrR in VPA-induced ASD rats. Our results suggested that both CT-DBS and 5-TH treatments were increased in the social interaction and functional connectivity in the bilaterally intrahemispheric M1-striatum circuits in the VPA-induced offspring. This study indicates cortico-striatal pathway play a potential therapy role in ASD.Acknowledgements
This research is financially supported by the Ministry Science and Technology of the Republic of China, Taiwan under Contract numbers of MOST 103-2320-B-010-014-MY2, 103-2321-B-010-016 and 102-2221-E-010-001-MY3 and the Zhenjiang University, China under the Fund number of 181110-193544B01/007.References
1. Loucas, T., Autism spectrum disorder. Supporting young children with communication problems, 2015:104. 2. Jeremy J. at al., Lamotrigine–valproic acid combination therapy for medically refractory epilepsy, Epilepsia, 2009, 50(3):475-479. 3. Rogawski, M. A. at al., The neurobiology of antiepileptic drugs, Nature Rev Neurosci, 2004, 5(7): 553-564. 4. Bromley, R. L., et al. Autism spectrum disorders following in utero exposure to antiepileptic drugs. Neurol, 2008, 71(23):1923-1924. 5. Rinaldi, T. et al., Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid. Cereb Cortex, 2008, 18(4):763-770. 6. Li, Xingbao, et al., Using interleaved transcranial magnetic stimulation/functional magnetic resonance imaging (fMRI) and dynamic causal modeling to understand the discrete circuit specific changes of medications: lamotrigine and valproic acid changes in motor or prefrontal effective connectivity. Psychiatry Research: Neuroimaging, 2011, 194(2):141-148. 7. Shirvalkar P. et al., Cognitive enhancement with central thalamic electrical stimulation, PNAS, 2006, 103:17007-17012 8. Lin, Hui-Ching, et al. Central thalamic deep-brain stimulation alters striatal-thalamic connectivity in cognitive neural behavior, Front neural circuits, 2016, 9 (87):1-12. 9. Hammock, Elizabeth, et al., Examining autism spectrum disorders by biomarkers: example from the oxytocin and serotonin systems, JAACAP, 2012, 51.(7): 712-721. 10.Wang C. C. et al., 5-HT1A-receptor agonist modified amygdala activity and amygdala-associated social behavior in a valproate-induced rat autism model. Int J Neuropsycho-pharmacol, 2013, 9:2027-2039. 11. Markram, K., et al. Abnormal fear conditioning and amygdala processing in an animal model of autism, Neuropsychopharmacol, 2008, 33(4): 901-912.Figures
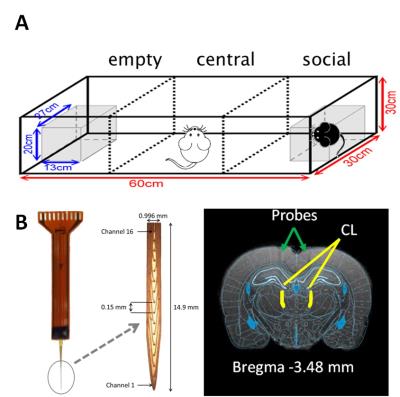
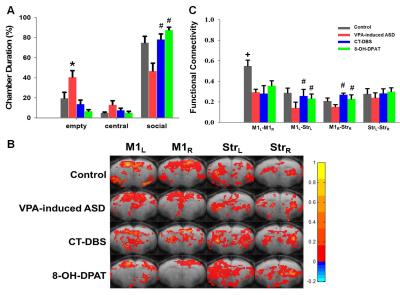
Fig 2. (A) Social interaction behavioral testing shows that both CT-DBS and 5-TH treatments could improve the social interaction. (B) Pattern comparison of the functional connectivity in four groups. (C) Statistical analysis of the functional connectivity shows that both CT-DBS and 5-TH treatments could modulate the motor cortico-striatal connectivity. The symbol * indicates significant difference as compared with the control. The symbol # indicates that both CT-DBS and 8-OH-DPAT treatments are significant difference as compared with the VPA-induced ASD in all ASD offspring. The symbol + indicates control group is significant difference as compared with the other groups. (P<0.05)