4071
MEMRI atlas-based assessment of brain volumes in adult NSG mice irradiated at birth1Radiology, University of Nebraska Medical Center, Omaha, NE, United States, 2Pharmacology & Experimental Neuroscience, University of Nebraska Medical Center, Omaha, NE, United States
Synopsis
Use of HIV-1 humanized mice allows the evaluation of brain morphology affected by disease and disease altering therapies. We posit that such studies can elucidate the pathobiology of HIV-1 associated neurological disorders. As irradiation, administered at birth, is a required step for long-term graft integrity we evaluated the effect of irradiation on mouse brain development using manganese-enhanced MRI (MEMRI) atlas-based segmentation. Brain size reductions in irradiated mice showed substantive morphological alterations. Thus, evaluation of irradiation effects on brain morphology is a requisite step in assessments of virus-induced brain pathology.
INTRODUCTION
Humanized NOD/scid-$$$\gamma_{c}^{null}$$$ (NSG) mouse models are widely used to study human immunodeficiency virus type one (HIV-1) amongst other disorders.$$$^{1,2}$$$ To generate these animals, mice are irradiated at birth which facilitates sustained engraftment of human immunocytes. Thus, a thorough understanding of the pathophysiological and morphological changes that occur by irradiation at birth provides a starting foundation to assess brain alterations affected by neurological disease. To this end, we studied the volumetric changes of brain regions in adult NSG mice that were irradiated at birth by segmenting brain structures using MEMRI mouse brain atlas.METHODS
Nineteen non-irradiated NSG mice (male, weight: 28.5 $$$\pm$$$ 2.4 grams, age: $$$\sim$$$1 year) that were originally used in MEMRI atlas generation were used as control NSG mice.$$$^3$$$ The NSG mouse brain MEMRI atlas is available at https://www.nitrc.org/projects/memribrainatlas/. Irradiation: Eight NSG pups after the first or second day of birth were irradiated either with a 55 s exposure equaling 1.1 Gy using RS-2000 X-ray irradiator (Rad Sourse Technologies, Inc.) or at 1Gy using a C9 cobalt 60 source (Picker Corporation). MRI acquisition: MEMRI was performed on irradiated mice at 1 year of their age (male, weight: 19.14 $$$\pm$$$ 0.54 grams, age: $$$\sim$$$1 year). Mice were injected with $$$MnCl_{2}$$$ (50Mm) 60mg/kg for 4 days. MRI data were acquired 24 hours after the last $$$MnCl_{2}$$$ administration using a Bruker Bioscan 7 Tesla/21 cm small animal scanner (Bruker, Billerica, MA) operating Paravision 5.1. An 82 mm actively decoupled volume resonator was used for signal transmission and a four-channel phase array coil was used for reception. Mice were anesthetized by inhalation of isoflurane in 100% oxygen and maintained 40-80 breaths/minute. Three-dimensional T$$$_1$$$-weighted spin echo images were acquired using RARE sequence with the parameters: TR/TE$$$_{eff}$$$ = 600/7.2 ms, RARE factor = 4, number of averages = 1, image matrix = 128 x 128 x 176 with 100 µm isotropic pixel size, total scan time = 27 min, anterior-posterior readout. Atlas-based segmentation: All MR brain volumes were manually extracted. Individual mouse brain MRI was registered using linear and Large Deformation Diffeomorphic Metric Mapping (LDDMM) nonlinear methods to the atlas MRI and image deformation matrix was computed. This deformation matrix was applied to the atlas to transfer labels onto the individual mouse MRI allowing segmentation and volume computation of brain structures. All image registration procedures were performed using Diffeomap 1.6v as implemented in DTIStudio software (www.mristudio.org).RESULTS and DISCUSSION
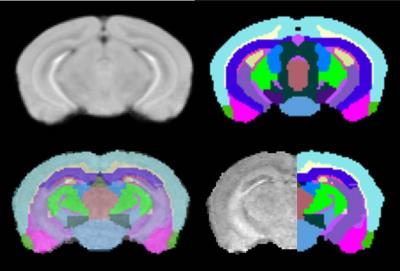
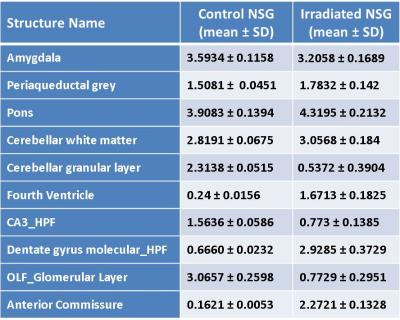
A representative slice of atlas and the corresponding color labels are shown (Top row, Fig. 1). For visual assessment of the quality of atlas-based segmentation, an overlay of transformed labels in transparent mode onto the MRI of one of the irradiated mice is shown (Left bottom, Fig. 1). MRI with labels on the right half (Right bottom, Fig 1) is displayed to demonstrate labeling accuracy and image contrast due to $$$MnCl_{2}$$$. Brain volume measurements were performed on irradiated (n=8) NSG mice and compared with control non-irradiated NSG (n=19) to determine irradiation-associated morphological brain alterations. Irradiated mouse brain volumes (436.26 $$$\pm$$$ 28.01 $$$mm^{3}$$$ ) were significantly (p<0.01) smaller compared with control NSG mouse brain volumes (503.63 $$$\pm$$$ 13.05 $$$mm^{3}$$$). This is in concurrence with their body weights. Majority of the individual structures’ volumes of irradiated mice are significantly smaller than their control counterparts. To remove the effect of brain size, each structure volume is normalized by the whole brain volume of corresponding mouse. Ten structures’ normalized volumes (%) (Table 1, mean$$$\pm$$$ SD) out of 41 segmented structures showed significant differences (p<0.01) between control NSG mice and irradiated NSG mice indicating variable effect of irradiation on different brain regions. It is known that the irradiation of mouse brain at birth has the effect of developmental delays on many structures.$$$^4$$$ Our results demonstrate that the volume changes occurring in the humanization process (both irradiation and cell engraftment combined)$$$^3$$$ is mainly due to irradiation with little or no contribution from human cell engraftment. For further understanding of the effect of irradiation at birth on adult brain, a longitudinal investigation of morphological, functional, and metabolic changes requires a larger animal group.CONCLUSION
Irradiation at birth has altered NSG mouse brain volume at one year of age. Understanding the pathophysiological processes involved in irradiation followed by cell engraftment in the humanization process of these mice show that irradiated mice with no cell engraftment provides a robust background for determining effects of HIV-1 infection on the brain.Acknowledgements
This work was supported, in part, by the University of Nebraska Foundation which includes individual donations from Carol Swarts and Frances and Louie Blumkin, the Vice Chancellor’s office of the University of Nebraska Medical Center for Core Facility Developments, Shoemaker Award for Neurodegenerative Research, ViiV Healthcare and National Institutes of Health grants K25 MH08985, P01 DA028555, R01 NS36126, P01 NS31492, 2R01 NS034239, P01 MH64570, P01 NS43985, P30 MH062261 and R01 AG043540.References
1. Victor Garcia J. Humanized mice for HIV and AIDS research. Curr Opin Virol. 2016 Aug;19:56-64.
2. Gorantla S, Gendelman HE, Poluektova LY. Can humanized mice reflect the complex pathobiology of HIV-associated neurocognitive disorders? J Neuroimmune Pharmacol. 2012;7(2):352-62.
3. Sajja BR, Bade AN, Zhou B, et al. Generation and Disease Model Relevance of a Manganese Enhanced Magnetic Resonance Imaging-Based NOD/scid- Mouse Brain Atlas. J Neuroimmune Pharmacol. 2016;11(1):133-41.
4. Gazdzinski L, Cormier K, Lu G, et al. Radiation-induced alterations in mouse brain development characterized by magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2012; 84(5):e631-8.
Figures