4048
Systematic Differences in High Angular Resolution Diffusion Imaging at Baseline in a Multicenter Longitudinal Clinical Trial1The Cleveland Clinic, Cleveland, OH, United States, 2Keller Center for Imaging Innovation, Barrow Neurological Institute, Phoenix, AZ, United States
Synopsis
The lack of imaging biomarkers is a key obstacle to the development of treatment for progressive multiple sclerosis. While diffusion MRI is a promising biomarker, variability among scanners may limit its use. We examine site-related variability among multiple sclerosis patients to inform the design of multicenter trials.
PURPOSE
There are no established treatments for progressive multiple sclerosis (MS). The lack of biomarkers for disease progression is one of the key obstacles to the development of treatment. The SPRINT-MS trial is a multicenter clinical trial of Ibudilast as a treatment for progressive multiple sclerosis (MS). One goal of this study is evaluation of advanced imaging metrics, including diffusion tensor imaging (DTI), as biomarkers for disease progression. Analysis of phantom scans has demonstrated differences among scanner platforms among the sites in this trial1. The purpose of this contribution is to determine if there are systematic differences among scanner platforms in vivo in a population of multiple sclerosis patients. The results are relevant for effective design for future trials.METHODS
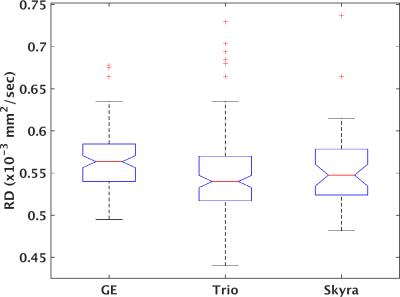
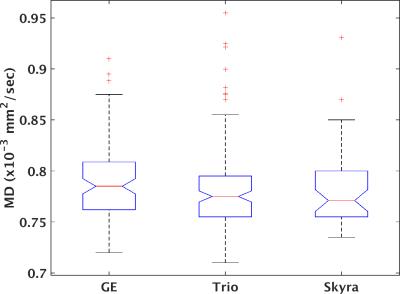
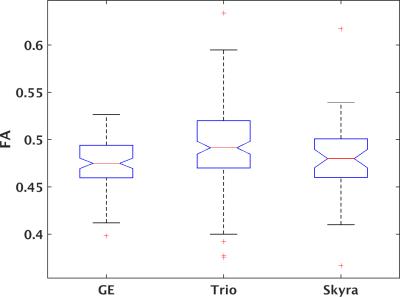
277 unique patients with progressive MS were recruited under an IRB-approved protocol within the NeuroNext network (https://www.neuronext.org) and were imaged on a 3 tesla GE (GE Healthcare, Waukesha) or Siemens (Siemens Healthcare, Erlangen) scanner with standard software and hardware at one of 27 sites. GE scanners consisted of seven Signa HDxt and one each of Signa Excite, Discovery MR750 and Discovery MR750W. Siemens scanners consisted of 6 Skyra and 11 Trio. High angular resolution diffusion imaging2 was acquired at 2.5mm isotropic resolution with 64 non-collinear diffusion weighting gradients with b = 700 sec/mm2 and 8 b = 0 images. Further details are provided in Zhou et al.1. Probabilistic tractography3 was performed to identify pyramidal tracts, and weighted means of tissue microstructure parameters (axial diffusivity (AD), radial diffusivity (RD), mean diffusivity (MD) and fractional anisotropy (FA)) within the tracks were calculated. Previous work indicated homogeneity of performance among GE scanners but marked differences between Siemens Trio and Skyra scanners. Tissue microstructure parameters were therefore divided into three groups: GE, Trio and Skyra. A one-way ANOVA with α = 0.05 was used to test for significant differences among groups, followed by a Tukey-Kramer post-hoc test using MATLAB (MATLAB Release 2015a, The MathWorks, Inc., Natick, Massachusetts, United States). No correction for multiple comparisons was performed.RESULTS
Figures 1-4 show boxplots of tissue microstructure among the imaging platforms. Significant differences were found for RD (p < 0.03) and FA (p < 0.0002) but not for AD (p > 0.08) or MD (p > 0.5). The post-hoc test showed that differences were significant between GE and Trio scanners for RD and FA.DISCUSSION
The results suggest that there are systematic differences among scanner platforms in DTI tissue microstructure parameters in a population of progressive MS patients. Substantial effort was placed in harmonizing the acquisitions across scanners, and a smaller study showed that such harmonization could effectively limit scanner-related biases4. However, this more extensive study in patients suggests that scanner-related biases are statistically significant. The analysis is limited by the assumption that disease- and population-related sources of variability are balanced among the scanner platforms.CONCLUSION
Studies characterizing differences among scanners are typically limited to healthy controls. It is important to characterize imaging outcomes based on the study population of interest, in this case progressive MS patients, because patterns of variability may differ between populations of healthy controls and disease-affected subjects. The detected variability supports the use of a longitudinal design to limit the impact of inter-scanner biases.Acknowledgements
We acknowledge support from NIH U01 NS082329 and NMSS RG-5184-A-6.References
1. Zhou, X. et al. Quantitative quality assurance in a multicenter HARDI clinical trial at 3T. Magn Reson Imaging 2016; 35:81-90.
2. Tuch, D. S. et al. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med 2002; 48(4):577-582.
3. Lowe, M. J. et al. Resting state sensorimotor functional connectivity in multiple sclerosis inversely correlates with transcallosal motor pathway transverse diffusivity. Hum Brain Mapp 2008; 29(7):818-827.
4. Fox, R. J. et al. A validation study of multicenter diffusion tensor imaging: reliability of fractional anisotropy and diffusivity values. AJNR Am J Neuroradiol 2012; 33(4):695-700.
Figures