4047
A longitudinal study of neurite orientation dispersion and density imaging in relapsing-remitting multiple sclerosisElda Fischi-Gomez1,2,3, Guillaume Bonnier1,2, Pavel Falkowskiy3,4,5, David Romascano3, Myriam Schluep6, Renaud Du Pasquier6, Alessandro Daducci3, Jean-Philippe Thiran3,4, Gunnar Kruger7, and Cristina Granziera1,2
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Department of Radiology, Charlestown, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Signal Processing Laboratory (LTS 5), Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 4Department of Radiology, Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 5Advanced Clinical Imaging Technology (HC CMEA SUI DI BM PI), Siemens Healthcare AG, Lausanne, Switzerland, 6Department of Clinical Neurosciences. Neuroimmunology and Neurology, Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 7Siemens Healthcare USA, Malvern, PA, United States
Synopsis
We explored the sensitivity of a novel diffusion MRI method i.e. “Neurite orientation dispersion and density imaging”, to detect and characterize brain microstructure alterations in relapsing-remitting multiple sclerosis patients that we followed up over 2 years. Cross-sectionally, NODDI revealed that an increase in orientation dispersion and a decrease in neurite density in NAWM and in lesions of RRMS patients compared to healthy subjects. Longitudinally, NODDI measured a decreased dispersion and an increased neurite density in MS lesions at 2 years follow-up. Also, NODDI metrics at baseline were highly related to cognition at both baseline and follow-up.
Purpose
Multiple sclerosis (MS) is a chronic inflammatory and degenerative disease of the central nervous system, characterized by the presence of focal and diffuse demyelination, gliosis and axonal damage in both white matter (WM) and grey matter (GM)1. Neurite orientation dispersion and density imaging (NODDI) is a recent diffusion imaging technique that uses diffusion gradients of different strengths to provide novel metrics of axonal and dendrites integrity2. Two previous preliminary works3,4 showed that NODDI is sensitive to detect brain structural damage in MS patients compared to controls. In this study, we explore the value of NODDI to investigate the longitudinal remodeling of axons and neurites in a population of early MS patients.Methods
Thirty-five RRMS patients with less than 5 years disease duration and 20 healthy controls (HC) were enrolled in the study (Table 1); 87% of patients were on treatment (high-dosage interferon beta and fingolimod) at study entry (time-point 1, tp1) and 97% at 2 years follow-up (time-point 2, tp2). All subjects underwent disability, motor and cognitive assessment (Table 2) and Magnetic Resonance Imaging (MRI) at both time-points (Table 3). We performed: (i) tissue segmentation and lobar parcellation on MPRAGE images using Morphobox5,6; (ii) manual lesion segmentation on 3DFLAIR, DIR and MP2RAGE images by two raters by consensus; (iii) MP2RAGE registration to the diffusion space and application of the resulting transformation matrix to all segmented volumes, masks and diffusion weighted images (DWI) volumes using Statistical Parametric Mapping toolbox7. To minimize partial volume (PV) effects, PV was estimated in each DWI by applying an iterative PV estimation algorithm to the b0 image8. DWIs were fitted to the NODDI model using the AMICO framework9 to extract the intra-cellular volume fraction (ICVF), neurite dispersion index (Orientation Dispersion Index (ODI)) and isotropic volume fraction (isoVF) in normal appearing WM (NAWM) and in lesions. To study the evolution of lesion properties, the lesion mask obtained at tp1 was registered to NODDI maps at tp2 and only lesions > 10 voxels were considered to minimize PV effects. At tp1, NODDI metrics in NAWM of each lobe were compared between RRMS patients and HC using the Hotelling two-sided permutation test (n=10000 permutations), with age and gender as covariates and correction for family-wise error rate. The same statistical analysis was used to compare mean NODDI metrics in lesions with mean NODDI metrics in the WM of HC. For longitudinal analysis we performed a t-test to assess differences between tp1 and tp2 in mean ICVF and ODI metrics in NAWM and lesions. At both time points, a GLM model was performed with mean ICVF and ODI measures at tp1 as predictors and clinical scores at tp1 and tp2 as random variables, with age, gender, educational years, anxiety and depression scores as covariates. Clinical scores were adapted using a box-cox transformation to satisfy the model assumption for normality. Bonferroni correction was applied to account for multiple comparisons.Results
Results for the cross-sectional comparison between patients and controls at tp1 are summarized in Table 4. The GLM analysis showed that (i) NAWM-ODI in the occipital lobe of tp1 was correlated to the SRT-LTS score (p=0.02, Adjusted-R2=0.32, Leave-one-Out Spearman (LOO-S)=0.43) and to the MSFC score (p=0.05, Adjusted-R2=0.3, LOO-S=0.37) at tp1; and (ii) NAWM ODI in the parietal and occipital lobes was correlated with the SRT-LTR score at tp2 (p=0.0018, Adjusted-R2=0.44, LOO-S=0.59). The combined analysis of ODI and ICVF variations in lesions showed a decreased dispersion and an increased neurite density at 2 years follow-up (Figure 1, p < 0,05).Discussion
NODDI appeared to be sensitive to detect cross-sectional differences between early RRMS patients and HC and longitudinal changes in patients. Cross-sectionally, MS patients exhibited an increase in orientation dispersion and a decrease in neurite density in NAWM and lesions compared to HC. The significant decrease of the ICVF may be due to axonal loss or damage, whilst the increase of the neural dispersion may result from cell and axonal loss resulting in increased extracellular space10. Longitudinal analysis showed subtle changes indicating an increase of neurite dispersion and of intraneurite volume in lesions, which suggest repair mechanisms in areas of focal damage11. Future work will extend the current findings to other MS subtypes and explore the value of NODDI in monitoring disease progression.Acknowledgements
No acknowledgement found.References
[1] Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. Sep 28;343(13):938-52, 2000. [2] Zhang, H., Schneider, T., Wheeler-Kingshott, C. A., & Alexander, D. C. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage, 61(4), 2012 [3] S. Corollone, N. Cawley, F. Prados, S. Ourselin, C. Tur GOMez, F. Grussu, C. G. Wheeler-Kingshott, D. Miller, A. Thompson, A. Toosy and O. Cicarelli. Neurite orientation dispersion and density imaging (NODDI) at the onset of clinically isolated syndroms (CIS): new insights in the early microstructural brain tissue changes. Neurology, 8 (16); Supplement P4.157, 2015 [4] W. Bronwlee, P. Alves Da Mota, F. Prados, T. Schneider, M. Cardoso, D. Altman, S. Ourselin, CG Wheeler-Kingshott, O. Cicarelli and D. Miller. Neurite Orientation Dispersion and Density Imaging (NODDI) Is Sensitive to Microstructural Damage Related to Disability in Relapse-Onset MS. Neurology, 8 (16); Supplement S41.003, 2015 [5] Roche A., Ribes D., Bach-Cuadra M., Krüger G. On the convergence of em-like algorithms for image segmentation using Markov random fields. Med. Image Anal. 15(6):830–839, 2011 [6] Schmitter D., Roche A., Maréchal B., Ribes D., Abdulkadir A., Bach-Cuadra M., Daducci A., Granziera C., Klöppel S., Maeder P., Meuli R., Krueger G., Alzheimer's Disease Neuroimaging Initiative An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer's disease. Neuroimage Clin. ,7:7–17, 2015 [7] http://www.fil.ion.ucl.ac.uk/spm/doc/ [8] Roche et al. Partial Volume Estimation in Brain MRI Revisited. Med Image Comput Comput Assist Interv. 2014.; [9] A. Daducci, E.J. Canales-Rodrigues, H. Zhang, T. Dirby, D.C. Alexander and J.-P. Thiran. “Accelerated microstructure imaging via convex optimization (AMICO) from diffusion MRI data.” NeuroImage 105:32-44, 2015. [10] M.H. Barnett, E. Mathey, M. C. Kiernan, J. D Pollard . Axonal damage in central and peripheral nervous system inflammatory demyelinating diseases: common and divergent pathways of tissue damage. Curr Opin Neurol Jun;29(3):213-21, 2016 [11] M. Absinta, P. Sati , DS Reich. Advanced MRI and staging of multiple sclerosis lesions. Nat Rev Neurol. 2016 Jun;12(6):358-68. doi: 10.1038/nrneurol.2016.59. Epub 2016 Apr 29.Figures
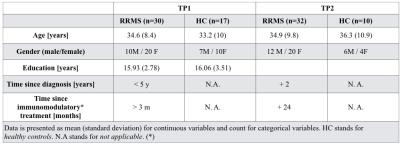
Table 1: Sample characteristics of the study
participants according to Relapsing-Remitting Multiple Sclerosis (RRMS)
diagnosis. At the moment of the study no patient had received corticosteroid therapy. *Immunomodulatory treatment consisted in high
dosage IFN beta or fingolimod.
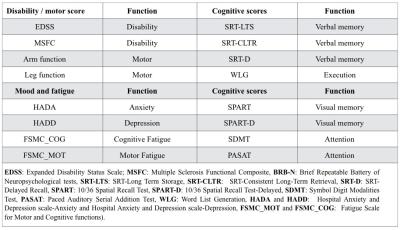
Table 2: Clinical test underwent for all subjects under
study
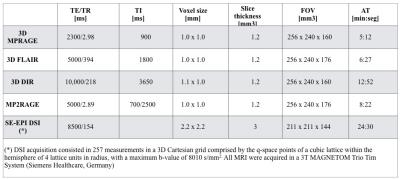
Table 3: Acquisition protocol at both time points
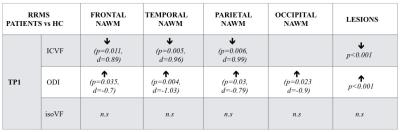
NODDI
metrics statistically significant differences found in the RRMS-HC comparison
for all subjects under study at the two different time-points. Arrows indicate
the sense of the difference (upward pointing arrow
indicates decreased and downward pointing arrow indicates increased values). The p-value (p) and the effect size of the
comparison (d) are shown in parentheses. n.s. stands for "not significative"
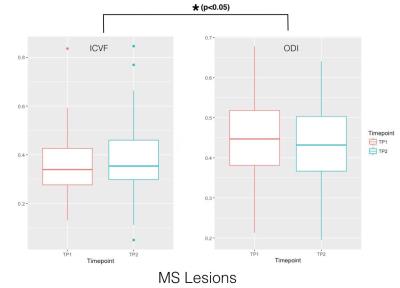
Boxplot for the
longitudinal evolution of the mean ICVF and ODI metrics computed over the
lesions higher than 10 voxels.