4036
A new ultrafast 3D gradient-echo magnetic resonance imaging method: RASE-I1Center for Neuroscience Imaging Research, Institute for Basic Science (IBS), Suwon, Korea, Republic of, 2Department of Biomedical Engineering Sungkyunkwan University (SKKU), Suwon, Korea, Republic of
Synopsis
One version of a new ultrafast gradient-echo-based 3D imaging technique using spatiotemporal encoding (RASE-I) is proposed which can provide very short TEs in some slices. RASE-I maintains most of appealing features of other spin-echo-based SPEN imaging methods including no Nyquist ghosting and high tolerance to field inhomogeneities. It is barely affected by T2* signal modulation and less sensitive to T2* effects due to local rephasing mechanism along the SPEN direction. Its performance is demonstrated by lemon and in-vivo mouse kidney imaging at 9.4T, including the measurement of dose-dependent arterial-input-function (AIF) of kidney-feeding artery.
Purpose
A new ultrafast gradient-echo-based 3D MRI is proposed using spatiotemporal encoding1,2 (SPEN), which is dubbed RASE (Rapid Acquisition with Sequential Excitation). Two versions of RASE (I and II) are available. One is to enable a much shorter effective-TE than EPI counter parts and the other provides constant TE across an object to be imaged. Here the first one (RASE-I) is introduced and demonstrated by lemon and in-vivo mouse imaging on a 9.4-T (Bruker-BioSpec, 94/30 US/R) animal scanner. As a promising application, it was also tested for DCE-MRI measuring the arterial-input-function (AIF) of kidney-feeding artery.Method
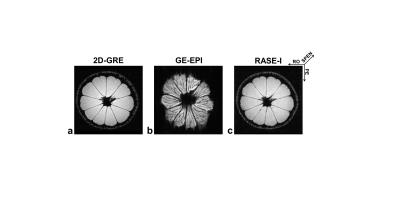
Figure 1 illustrates the sequence diagram of RASE-I. A frequency-swept chirp pulse is used for sequential spin excitation which produces a quadratic phase that localizes a signal in both time and space in the presence of gradient. In RASE-I, spin excitation is performed with a much shorter duration than data acquisition by using a shortened chirp pulse (≤ 1ms), which allows very short TEs in some slices defined by early rephasing spins. During the data acquisition, spin isochromats are sequentially and locally rephased but in reverse order of sequential excitation by applying another gradient with opposite polarity to the slab-selective gradient. The slab-selective direction is spatiotemporally encoded3, which makes the spins of the same slice experience the same TE unlike EPI. Images were reconstructed offline with MATLAB (ver.8.2.0; R2013b) using the super-resolution algorithm4. For lemon imaging, 2D-GRE, GE-EPI, and RASE-I sequences were used for comparison. Same scan parameters were: FOV = 65 × 65mm2, matrix = 128 × 128, number of slices = 48, slice thickness (THK) = 0.3125mm. In GRE imaging as a reference, TR/TE = 310/3ms, flip angle (FA) = 33°. In GE-EPI imaging, shot-TR/TE = 3,600/36.6ms, FA = 90°. In RASE-I imaging, shot-TR/TE = 57/(1.9~26.4)ms, FA = 14.3°, R-value (pulse_length × bandwidth) = 256. To validate the feasibility of RASE-I for DCE-MRI applications, dose-dependent AIF of kidney-feeding artery was measured in in-vivo mice at three different injection doses of Gd-DOTA(0.1, 0.2, and 0.3 mmol/kg, n = 2 each). For estimation of absolute Gd-concentration, signal intensity was converted to R1 using the following equations5,6:
$$R_{1}\left(t\right)=\frac{1}{TR}\ln\left(\frac{S_{0}\sin(FA)e^{\frac{TE}{T_2^*}}-S(t)\cos(FA)}{S_{0}\sin(FA)e^{\frac{TE}{T_2^*}}-S(t)}\right)$$ [1]
$$S_{0}=S_{ss}\frac{1-cos(FA)e^{\frac{TR}{T_{1,0}}}}{(1-e^{\frac{TR}{T_{1,0}}})sin(FA)e^{\frac{TE}{c_2^*}}}$$ [2]
where Sss and S(t) are pre- and post-injection signal intensities, respectively. T2* contribution was ignored due to short TE. R1 was then converted to Gd-concentration using5:
$$\frac{1}{T_{1}}=r_{1},Gd-DOTA\times C_{p}\times\left(1-H_{ct}\right)+\frac{1}{T_{1,0}}$$ [3]
where r1,Gd is T1 relaxivity of Gd-DOTA (= 4.156 s-1·mM-1), Cp is Gd-concentration in plasma. Hct is a portion of hematocrits (= 0.45)7. T1,0 and T1 are pre- and post-injection relaxation times, respectively. For RASE-I imaging, scan parameters were: TR/TE = 21/(2~18) ms, pulse duration = 0.7ms, FA = 35°, FOV = 35×35mm2, matrix = 96×96, THK = 0.3125mm, temporal resolution = 2.16s, number of slices = 48, and R-value = 256. In TSE imaging for T1,0 and T1 measurements, TE = 5.07ms, TR = 110 to 5,000ms, turbo factor = 4. T1,0 values at the artery region were averaged in six mice to be 1.5s. Total acquisition time was 10min 4.8s and contrast agent was injected 1 minute before acquisition.
Results
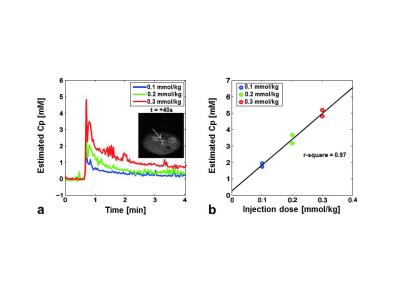
In Fig.2, since the shortest TE slice was representatively presented, RASE-I showed a much better image quality with less distortion than GE-EPI because of higher tolerance to field inhomogeneities due to larger gradient amplitudes, very shorter TE, and less T2* effects due to local rephrasing mechanism in the SPEN direction. The estimated AIFs at the injection dose of 0.1, 0.2, and 0.3 mmol/kg are shown in Fig.3a as blue, green, and red lines, respectively. The subfigure in Fig.3a shows the representative artery region (white arrow) 40s after injection. The peak concentration of in-vivo AIF linearly increased as the injection dose increased, validating the accuracy of Gd-concentration estimation with dynamic RASE-I imaging. In Fig.3b, the peak Gd-concentrations of AIFs at three injection doses were scatter-plotted, showing an apparent linear correlation (r2=0.97, P<0.001).Discussion & Conclusion
One version of new gradient-echo-based 3D ultrafast imaging method (RASE-I) was presented here. RASE-I maintains most of appealing features of other spin-echo-based SPEN imaging methods including no Nyquist ghosting in the SPEN direction and higher tolerance to image distortions due to field inhomogeneities than EPI counterparts1, 2. RASE-I is also able to provide very short TEs in some slices which are early rephrased. As a promising application, RASE-I can be used for accurate quantification of absolute Gd-concentration even at high doses in DCE-MRI, providing extended volume coverage and minimized inflow effects8 due to its 3D characteristics as well as less sensitivity to T2* effects due to local spin-rephasing mechanism.Acknowledgements
This work was supported by IBS-R015-D1References
[1] Camberlain R et al., Mag Reson Med 2007;58:794-799. [2] Ben-Eliezer N et al., Mag Reson Imaging. 2010;28:77-86. [3] Ryu JK et al., ISMRM. 2015;6897. [4] Chen Y et al., Mag Reson Med. 2013;69:1326-1336. [5] Landis CS at al., Magn Reson Med 2000;44:563-574. [6] Han SH et al., J Magn Reson Imaging 2016;44:138-147. [7] Raaba BM at al., J Am Assoc Lab Anim SciJAALAS 2011;50:680. [8] Gao J.H at al., Neuroimage. 2012;62:1035-1039.Figures