4024
Ultra-short Echo-time MRI Lung Segmentation using High-Dimensional Features and Continuous Max-Flow1Robarts Research Institute, London, ON, Canada, 2General Electric Healthcare, Milwaukee, WI, United States
Synopsis
Ultra-short-echo-time MRI may be used to generate imaging biomarkers to phenotype pulmonary abnormalities and facilitate the development of novel treatments but requires clinically-acceptable lung segmentation. We proposed an adaptive kernel K-means approach combining MRI signal intensity and neighbourhood location information for optimized lung segmentation. The resultant high dimensional features were implemented using a K-nearest neighbour graph and relaxed to a point-wise upper-bound formulation regularized by image edge information, which was implemented iteratively using a continuous max-flow optimization approach. Experimental results for 10 asthmatics demonstrated highly accurate, reproducible and computationally efficient lung segmentation for our approach consistent with clinical workflows.
Purpose
Ultra-short echo-time (UTE) MRI may be acquired on conventional scanners and provides enhanced visualization of lung structure and function. This technique is ideally suited for serial and longitudinal evaluation of pediatric patients and young adults as it does not require ionizing radiation or exogenous contrast.1 Previous studies1-3 have demonstrated the potential of UTE MRI biomarkers to phenotype lung structural-functional abnormalities and perhaps facilitate the development of novel lung disease treatments. To generate these imaging biomarkers and enable the widespread clinical application of UTE MRI, as a first step, accurate, reproducible and rapid lung segmentation methods are required. However, UTE MRI lung segmentation is particularly challenging because of inhomogeneous signal intensities, magnetic susceptibility and motion artefacts,4 dramatic changes of lung shapes and protruding structures.5 Previous studies6 have shown that optimal pulmonary MRI segmentation cannot be achieved using gray-level information exclusively. Therefore, the objective of this study was to develop an improved segmentation approach by incorporating high-dimensional features for optimal UTE MRI lung segmentation.Methods
Subjects and image acquisition:
To test our segmentation approach, a data-set of UTE lung images was acquired in 10 asthma patients that provided written informed consent to an ethics-board-approved protocol. UTE MRI was performed at 3.0T (Discovery MR750 system, General Electric Health Care, Milwaukee, WI) using a 32-channel torso coil and a proprietary research 3D cones UTE sequence.2 Coronal image acquisition was performed during inspiration breath-hold of 1.0L medical grade nitrogen from functional residual capacity (15s acquisition time; repetition time/echo time/flip angle=3.5ms/0.03ms/5°; field-of-view=40cm×40cm; matrix=200×200; 18 slices and 10mm slice thickness), as previously described.2
High-dimensional features, bound relaxation and continuous max-flow optimization:
We employed an adaptive kernel K-means approach7 to compensate for the inherently heterogeneous MRI lung signal intensities and preserve the continuity of lung segmentation. In addition, we incorporated neighbourhood spatial location information to aid in lung segmentation, resulting in high-dimensional features combining signal intensity and neighbourhood spatial location information. The adaptive kernel K-means clustering approach was implemented in the form of a K-nearest neighbour graph. The resultant high-order data term was relaxed to a point-wise formulation by deriving its upper bound7 to avoid tackling the original optimization problem directly. The regularization term was generated using image edge information.5 The constructed data and regularization terms were entered into a two-label continuous max-flow optimization approach8 to derive the optimal solution. The resultant objective function was implemented in an iterative manner where the current solution was carried to the next iteration to update the data term. The original challenging segmentation is guaranteed to converge because of the iterative implementation of the upper bound formulation. Prior to algorithm implementation, the original UTE images were resampled to ~2mm isotropic voxel size, and one observer seeded the lung and background on a single slice three times on three different days.
Validation:
Algorithm segmentation accuracy was evaluated by comparing algorithm lung masks with manual outputs generated by an experienced observer. We employed the Dice similarity coefficient (DSC), root-mean-squared-error (RMSE) of the distances between two lung surfaces and absolute percent volume error (|dVp|) as region-, surface distance- and volume-based similarity measurements for algorithm and manual results. Algorithm segmentation reproducibility was evaluated by calculating the Coefficient of Variation (CoV) and Intra-class Correlation of Coefficient (ICC) for DSC and RMSE. The computational efficiency of our approach was measured using run time.
Results
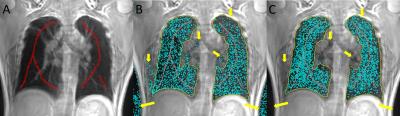
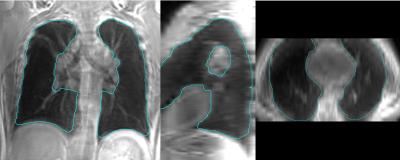
Table 1 provides a summary of the demographic characteristics for the 10 asthma patients. Representative UTE MRI lung and voxel labelling probability maps are shown in Figure 1. Coronal, axial and sagittal views of example UTE lung segmentation results are provided in Figure 2. For 10 asthma patients, our approach yielded a DSC of 92.8±2.5%, RMSE of 2.9±0.6 mm and |dVp| of 7.2±3.6%. CoV(ICC) were 0.4%(0.98) and 1.6%(0.97) for DSC and RMSE, respectively. The mean run time for our approach was ~2 min using a GPU including ~5s for user seeding.Discussion
Our approach avoids over-fitting of appearance models generated from user-seeds and algorithm-segmented lung. The incorporation of neighbourhood spatial location information further improved algorithm segmentation by favoring compact segmentation regions. As a result, the proposed approach required less user seeding and outperformed current methods using only fixed signal intensity models to describe the object of interest.Conclusion
Our approach involves diminished user interaction, generates highly reproducible lung segmentation with high computational efficiency consistent with clinical workflows. All of this is consistent with the need for widespread UTE MRI clinical applications. Future work will involve algorithm automation, vessel and airway segmentation, and software package integration for commercialization.Acknowledgements
We thank Trevor Szekeres, RTMR and Dave Reese, RTMR for MRI of research volunteers.References
1 Ma, W. et al. Ultra-short echo-time pulmonary MRI: Evaluation and reproducibility in COPD subjects with and without bronchiectasis. Journal of Magnetic Resonance Imaging 41, 1465-1474 (2015).
2 Sheikh, K. et al. Ultrashort echo time MRI biomarkers of asthma. Journal of Magnetic Resonance Imaging (2016).
3 Walkup, L. L. et al. Quantitative magnetic resonance imaging of bronchopulmonary dysplasia in the neonatal intensive care unit environment. American journal of respiratory and critical care medicine 192, 1215-1222 (2015).
4 Bergin, C., Pauly, J. & Macovski, A. Lung parenchyma: projection reconstruction MR imaging. Radiology 179, 777-781 (1991).
5 Guo, F. et al. Anatomical pulmonary magnetic resonance imaging segmentation for regional structure-function measurements of asthma. Medical Physics 43, 2911-2926 (2016).
6 Osareh, A. & Shadgar, B. A segmentation method of lung cavities using region aided geometric snakes. Journal of medical systems 34, 419-433 (2010).
7 Tang, M., Ben Ayed, I., Marin, D. & Boykov, Y. in Proceedings of the IEEE International Conference on Computer Vision. 1555-1563 (2015).
8 Yuan, J., Bae, E. & Tai, X.-C. in Computer Vision and Pattern Recognition (CVPR), 2010 IEEE Conference on. 2217-2224 (IEEE).
Figures