4022
2D Zero TE Imaging Implemented with Gradient-Extended 2D Spiral-in Excitation and Spiral-out Acquisition (GRESS-ZTE)1Center for Brain Imaging Science and Technology, Department of Biomedical Engineering, Zhejiang University, Hangzhou, Zhejiang, People's Republic of China
Synopsis
A 2D zero TE imaging technique was implemented with a gradient-extended 2D spiral-in excitation to excite a slice within 3mm full-width-half-maximum (FWHM) with pulse duration less than 6ms and spiral-out acquisition (GRESS-ZTE) strategy. Our preliminary data showed its ability to acquire signals in bones with very short T2*.
Purpose
2D spiral-in excitation trajectory is self-refocusing which means the echo time starts immediately at the end of the excitation pulse. Slice selection with a 2D spiral-in trajectory requires high frequency excitation k-space traversal, resulting in long pulse duration that is inefficient for fast imaging sequences and sensitive to B0 inhomogeneity. To effectively travel the high frequency excitation k-space, we proposed a gradient-extended strategy by taking advantage of 2D spiral excitation and SINC excitation. Zero TE imaging was implemented by combining the gradient-extended spiral-in excitation with spiral-out acquisition (GRESS-ZTE).Methods
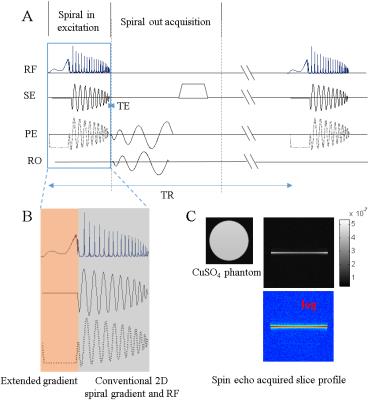
The GRESS-ZTE pulse sequence was shown in Fig. 1 (A). It was implemented on a Siemens 3T scanner equipped with 2 independent parallel transmission channels and a 20-channels receiving coil. For the excitation part, a spiral-in 2D RF pulse was designed with a thin slice as the 2D target excitation profile. The RF pulse was designed with a spatial domain based multidimensional RF design method 1. For the acquisition part, a 30 interleaves spiral-out acquisition with spatial resolution 1.3 mm and acquisition FOV 22 cm was performed. All the spiral waveforms in Fig. 1 were optimized with the fast gradient optimization method. After acquisition, a spoil gradient was applied along slice selection direction to eliminate the transverse magnetizations.
The proposed gradient-extended solution for GRESS-ZTE was shown in Fig.1 (B) where the excitation k-space was covered by the 2D spiral-in excitation at the k-space center and traditional SINC-like excitation at the high frequency k-space. The gray part in the Fig.1 (B) was the conventional 2D spiral excitation trajectory, which slowly covered the k-space with oscillating gradient. Even with the variable density spiral gradient, the k-space covering speed was limited by the oscillating property. While in the traditional SINC excitation, the k-space (kz) was uniformly and fast covered with a single polarized (positive/negative) gradient. Therefore, a single polarized gradient in the front of a low-resolution 2D spiral trajectory was used in this study. It is noted that although half SINC excitation has been previously applied in UTE imaging, it requires twice the imaging time to avoid the unwanted imaginary part of slice selection 2,3. But in our implementation of GRESS-ZTE there is no such need. Fig.1 (C) are the spin echo acquired excitation slice profiles in both gray scale and log scale of a cylinder CuSO4 phantom excited with a 5.62ms pulse including 1.48ms extended gradient and 4.14ms 2D spiral-in gradient.
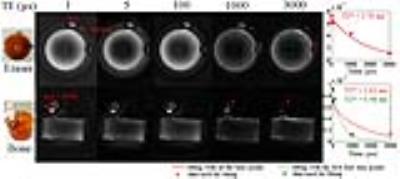
A PVP phantom with two erasers and a pig tube bone were imaged with flip angle = 30°, TR = 50ms and a variable echo time at 1, 5, 100, 1000 and 3000us. Images were reconstructed with NUFFT package 4 with MATLAB 2012b.
Results and Discussions
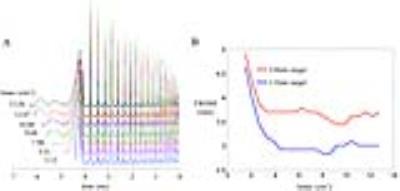
Fig. 2 shows the impacts of extended gradient on the designed RF waveforms and the excitation performance. The time integral of extended gradient and spiral-in gradient over the pulse duration has been calculated as kmax (cm-1). As the kmax increased, the SINC-like RF waveforms keep extending, while the 2D RF waveforms almost remain unchanged. The impact of kmax on the full-width-half-maximum (FWHM) of the excitation profile was quantified via Bloch simulation shown in Fig. 2 (B). The slice FWHM has an exponential-like decrease at the beginning of kmax, but starts to fluctuate when the SINC-like RF across the zero-crossing point.
Fig. 3 shows the eraser and bone images acquired with GRESS-ZTE. The exponential fittings of the signal from a small chosen ROI on the eraser and bone are the red lines shown at the right side of Fig. 3. T2* relaxation time of eraser and bone was found to be 1.70 ms and 1.63 ms respectively. The bone’s T2* time exceeds the reported value of hundreds of microseconds 5,6. Since the bone signal acquired at TE=3 ms was less than 5% intensity and strongly influenced by noise, the fitting was recalculated by excluding this time point. The new fitting curve is the green line shown in Fig. 3. The recalculated T2* value is 552 µs which is in agreement with the previous reports 5,6. The signal fluctuation at the start of acquisition may be due to the system imperfection including hardware delay and analog-to-digital (ADC) fluctuation.
Conclusion
In this study, we have proposed a GRESS-ZTE imaging technique, and our preliminary data showed its ability to acquire very short T2* signals. The imaging resolution and efficiency remain to be further optimized, and this method may find potential application in short T2* imaging including bone image.Acknowledgements
References
1. Grissom W A, Yip C, Zhang Z, et al. Spatial domain method for the design of RF pulses in multicoil parallel excitation. Magnetic Resonance in Medicine, 2006, 56(3): 620-629.
2. Du J, Carl M, Bydder M, et al. Qualitative and quantitative ultrashort echo time (UTE) imaging of cortical bone. Journal of Magnetic Resonance, 2010, 207(2): 304-311.
3. Sheth V, Shao H, Chen J, et al. Magnetic resonance imaging of myelin using ultrashort Echo time (UTE) pulse sequences: Phantom, specimen, volunteer and multiple sclerosis patient studies. NeuroImage, 2016: 37-44.
4. Fessler J A. On NUFFT-based gridding for non-Cartesian MRI. Journal of Magnetic Resonance, 2007, 188(2): 191-195.
5. Sun Y, Ventura M, Oosterwijk E, et al. Zero Echo Time Magnetic Resonance Imaging of Contrast-Agent-Enhanced Calcium Phosphate Bone Defect Fillers. Tissue Engineering Part C-methods, 2013, 19(4): 281-287.
6. Wehrli F W, Fernandezseara M A. Nuclear magnetic resonance studies of bone water. Annals of Biomedical Engineering, 2005, 33(1): 79-86.
Figures