4019
Spiral MPRAGE Acquisition and Segmentation1Department of Electrical Engineering, University of Hawaii at Manoa, Honolulu, HI, United States, 2Department of Medicine, John A. Burns School of Medicine, University of Hawaii, Honolulu, HI, United States
Synopsis
High-resolution MPRAGE has become a standard structural imaging sequence in clinical settings yet it is difficult to repeat the scan because of its relatively long acquisition time. Acceleration techniques allow the reduction of acquisition times although excessive acceleration can result in artifacts. We implemented a fast MPRAGE sequence using a spiral trajectory and demonstrated segmentation of acquired images. The spiral MPRAGE can acquire a whole-brain T1 volume in less than 25% of the acquisition time of the conventional MPRAGE while maintaining comparable contrast and segmentation results.
INTRODUCTION
High-resolution MPRAGE has become a standard structural imaging sequence in clinical settings yet it is difficult to repeat the scan because of its relatively long acquisition time. Acceleration techniques allow the reduction of acquisition times although large accelerations can result in artifacts. In this study, we implemented a fast MPRAGE sequence using a spiral trajectory and demonstrated segmentation of acquired images.METHODS
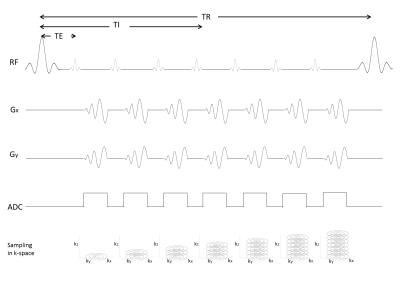
Spiral MPRAGE was implemented based on a standard MPRAGE design1, 2 and parameters which are widely used by clinicians. The pulse sequence design is shown in Figure 1. Between the 180º inversion recovery pulses, each spiral trajectory acquires 2048 readout points after each 10º alpha pulse for the entire set of 176 phase encodes along z. Each set of inversion recovery and z phase encoding is repeated to fill k-space for the number of spiral interleaves . We choose 1.4 ms for the value of TE to allow TI around 1000 ms to produce good contrast between white matter (WM) and gray matter (GM).
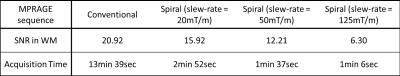
All scans were acquired on a 3 T whole-body Tim TRIO MR scanner (Siemens Healthcare, Erlangen, Germany) with a matrix coil array. One healthy subject volunteered for this study after providing informed consent. Three sets of spiral MPRAGE volumes were acquired with three different slew-rates (20, 50, and 125 mT/m) to adjust readout time and, consequently, the image signal-to-noise ratio (SNR). The acquisition times were 2 min 52 sec, 1 min 37 sec, and 1 min 6 sec. One conventional MPRAGE scan was also performed and its acquisition time was 13 min 39 sec. For a fair comparison, all the volumes were acquired with a 1x1x1 mm voxel size, a 256x256 encoding matrix, 176 partitions, and no acceleration.
The conventional MPRAGE volume was reconstructed online. The three spiral MPRAGE volumes were reconstructed offline with a non-uniform fast Fourier transform reconstruction3 with MATLAB (R2015b, Mathworks, Natick, MA, USA). After the images were reconstructed, the coordinate information was read from the header of the raw data and transformed to the reconstructed image volume. A voxel to RAS (vox2ras) transformation was necessary in order to properly process with segmentation software. Also, global scaling was applied to set the intensities of brain tissues to a range that is appropriate for the segmentation software. The four volumes were then segmented using FSL FAST4 and FreeSurfer5, 6. FreeSurfer WM volumes were used to calculate SNR.
RESULTS
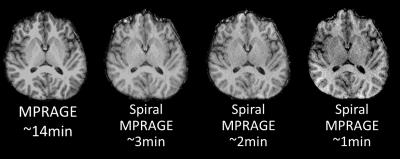
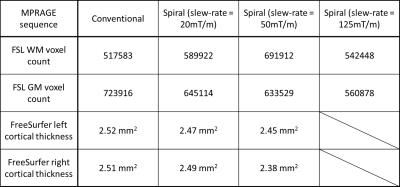
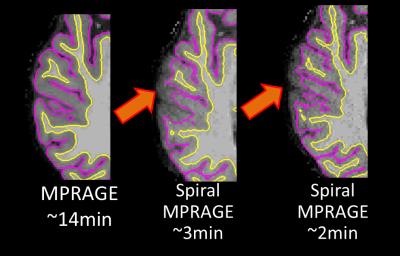
The whole-volume T1-weighted images were successfully acquired with spiral MPRAGE with the three different acquisition times. The acquisition time with respect to slew-rate and SNR are shown in Table 1. Compared to the significant reduction in acquisition time, SNR loss is modest. All spiral volumes had good image contrast between WM and GM, although the images became noticeably noisy with the fastest spiral MPRAGE acquisition (Figure 2). FreeSurfer completed segmenting all four volumes; however, there were segmentation errors in both WM and GM in the fastest spiral MPRAGE volume. The FSL voxel counts and FreeSurfer cortical thickness results showed that the conventional MPRAGE has more volume in GM (Table 2). FreeSurfer surfaces showed that the GM/cerebrospinal boundaries were underestimated in the spiral MPRAGE volumes (Figure 3).DISCUSSION
The contrast similarity between the conventional and spiral MPRAGE images encourages further investigation into the contrast-to-noise ratio. The underestimation of GM in the spiral volumes can also be investigated with the boundary displacements. From a visual comparison, ventricles looked a little larger in spiral images compared to the ones in the conventional MPRAGE volume. Comparing volumes of subcortical segmentation and cortical parcellation will help determining such differences. The use of different bias correction on the spiral MPRAGE images before running segmentation might also provide a more accurate analysis.CONCLUSION
The spiral MPRAGE can acquire a whole-brain T1 volume in less than 25% of the acquisition time of the conventional MPRAGE while maintaining comparable contrast and segmentation results. Fine-tuning of scan parameters and improved bias correction can make spiral MPRAGE competitive with the conventional MPRAGE.Acknowledgements
Grant # R01DA019912, R01EB011517, K02DA020569References
1. Mugler JP and Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE), Magnetic Resonance in Medicine 1990; 15, 1, 152-157.
2. Mugler JP and Brookeman JR. Rapid Three-dimensional T1-weighted MR Imaging with the MP-RAGE Sequence. Journal of Magnetic Resonance Imaging 1991;1: 561-567.
3. Fessler JA. On NUFFT-based gridding for non-Cartesian MRI. Journal of Magnetic Resonance 2007;188( 2):191-195.
4. Zhang, Y. and Brady, M. and Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imag 2001;20(1):45-57.
5. Dale A, Sereno M, and Fischl B. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999; 9(2):179-194.
6. Fischl B, Sereno M, and Dale A. Cortical surface-based analysis II. Inflation, Flattening, and a Surface-Based Coordinate System. Neuroimage 1999;207:195-207.
Figures