3972
3D Dual-Echo Dixon Turbo Spin Echo Imaging of the Spine using Compressed Sensing1Philips Research, Hamburg, Germany, 2UT Southwestern Medical Center, Dallas, TX, United States, 3Philips Healthcare, Hamburg, Germany, 4Leiden University Medical Center, Leiden, Netherlands
Synopsis
The acceptance of 3D TSE sequences in spine imaging has been low up to now, mainly because of their long scan times. In this work, their acceleration by a combination of compressed sensing and parallel imaging was investigated. Moreover, their extension by an integration of chemical shift encoding was explored to obtain two contrasts simultaneously. For this purpose, a 3D dual-echo Dixon TSE sequence was implemented and evaluated in T2-weighted imaging of the lumbar spine.
Introduction
Two-dimensional (2D) turbo spin echo (TSE) sequences are widely used in spine examinations today to image multiple parallel slices with high in-plane resolution. To mitigate the low through-plane resolution, they are often repeated in a different orientation. Three-dimensional (3D) TSE sequences promise to provide thinner slices with sufficient signal-to-noise ratio (SNR) and to permit a retrospective reformatting or alignment of the slices into any orientation or with any anatomical structure, without loss of resolution. However, despite considerable advances in recent years, their scan time still remains too long for an adoption into clinical routine, in particular in view of their coverage of a single contrast only. Therefore, the purpose of this work was to explore the basic feasibility of applying compressed sensing (CS) in addition to parallel imaging (PI) for further acceleration and of integrating chemical shift encoding for a simultaneous acquisition of two contrasts. A 3D dual-echo Dixon TSE sequence with pseudo-random phase encoding was developed for this purpose, and it was evaluated in T2-weighted imaging of the lumbar spine with and without fat suppression.Methods
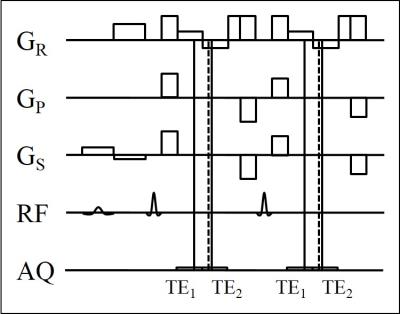
The proposed 3D dual-echo Dixon TSE sequence is illustrated in Fig. 1. Two gradient echoes are acquired around each spin echo with echo shifts of TE1/TE2. They are generated with two rephasing lobes of the readout gradient with opposite polarity, and they are sampled only partially towards the spin echo (kx,max < - kx,min) to decrease both, the TSE echo spacing ΔTETSE and the Dixon echo spacing ΔTEDIXON = TE2 - TE1.1 A flyback lobe is inserted after the rephasing lobes to satisfy the Carr-Purcell-Meiboom-Gill (CPMG) condition, and a pair of crusher lobes is added for spoiling of the free induction decay (FID) following each refocusing pulse. The flip angle of the refocusing pulses is varied over the TSE train, and the pairs of dephasing and rephasing lobes of the phase encoding and slab selection gradients are gradually rescaled to obtain a pseudo-random sampling of the ky-kz plane with variable density.
Healthy volunteers were examined with this sequence on a 3 T Ingenia scanner (Philips Healthcare, Best, Netherlands) using the integrated 12-element posterior coil array. T2-weighted images of the lumbar spine were acquired in sagittal orientation with a FOV of 280 (AP) x 280 (FH) x 70 (RL) mm3 and a resolution of 0.9 x 1.0 x 1.3 mm3. Oversampling in phase encoding and slab selection direction served the suppression of undesired aliasing from outside the FOV. A TE/TR of 168/1300 ms were used, and 64 spin echoes with a ΔTETSE of 7 ms were generated after each excitation. The data collected with TE1/TE2 were separately processed with an L1 norm-based CS reconstruction relying on pre-calibrated coil sensitivities.2-5 The resulting images were subjected to a two-point Dixon method for water-fat separation, which included a fat shift correction.6,7 As reference, images were also acquired with a 3D TSE sequence with the same parameters but without chemical shift encoding, using once PI and once a combination of CS and PI (CS-PI) for acceleration.
Results
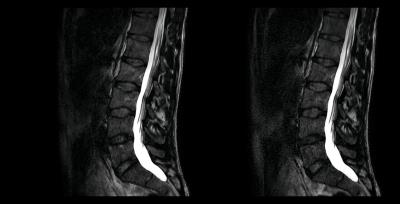
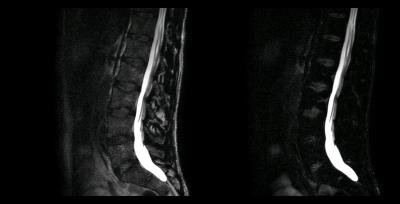
Selected results from one of the subjects are summarized in Figs. 2 and 3. Images produced with the 3D TSE sequence without chemical shift encoding are shown in Fig. 2. The scan time was 5:40 min with a 1.9-fold reduction by PI and 3:45 min with a 2.9-fold reduction by CS-PI in this case. Corresponding images obtained with the 3D dual-echo Dixon TSE sequence are provided in Fig. 3. The scan time was again 3:45 min with a 2.9-fold reduction by CS-PI. A TE1/TE2 of -1.2ms/0.2ms and a partial echo factor of 0.8 were employed in this case.Discussion
The use of a 50% higher acceleration in conjunction with CS decreased the scan time of the 3D TSE sequence well below five minutes. It led to a discernible loss in SNR in the original thin slices, which may still be acceptable, in particular in synthesized thicker slices. The acquisition of two partial gradient echoes instead of one spin echo was possible without increasing ΔTETSE. While the water images suffered only slightly from the associated higher bandwidth due to an averaging effect in the water-fat separation, the in-phase images deteriorated substantially in terms of SNR. Further optimization of the sequence and the reconstruction are required to improve their quality.Acknowledgements
The authors thank Drs. Beck and van Yperen for providing advice on the implementation of the 3D dual-echo Dixon TSE sequence.References
1. Wang X, et al. Proc ISMRM 2016; 573. 2. Zhao C, et al. Proc ISMRM 2008; 1478. 3. Wu B, et al. Proc ISMRM 2008; 1480. 4. King KF. Proc ISMRM 2008; 1488. 5. Liu B, et al. Proc ISMRM 2008; 3154. 6. Eggers H, et al. Magn Reson Med 2011; 65:96-107. 7. Lu W, et al. Magn Reson Med 2008; 60:198-209.Figures