3968
Improved Pulmonary Artery Non-Contrast Balanced Steady State Free Precession MR Imaging using Golden Angle Radial versus Cartesian Sampling1Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden, 2Department of Clinical Physiology, Karolinska University Hospital, Stockholm, Sweden, 3Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden, 4Department of Radiology, Karolinska University Hospital, Stockholm, Sweden, 5Department of Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Gothenburg, Sweden, 6Department of Radiation Physics, The Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden
Synopsis
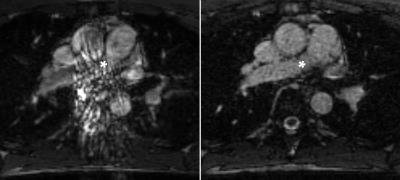
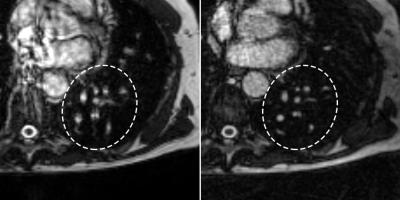
Free-breathing steady-state free precession MRI has shown promising results for pulmonary embolism diagnosis in preliminary studies. However, the acquisition is susceptible to artifacts from cardiorespiratory motion and flow. We propose a Golden Angle radial trajectory and show increased robustness to such artifacts in healthy volunteers, while providing added benefits such as sliding window reconstructions with higher temporal resolution than what is achievable by Cartesian sampling.
Purpose
Imaging pulmonary arteries with MRI has several benefits over contrast enhanced computed tomography angiography (CTA), which is the current modality of choice for evaluating pulmonary embolism (PE).1 Other than avoiding the use of ionizing radiation, studies suggest as many as 25% patients with suspected PE have contraindications for CTA, e.g. renal dysfunction.2 Non-contrast enhanced free breathing balanced steady state free precession MRI with Cartesian k-space sampling has been shown to have a high sensitivity and specificity for diagnosing PE albeit with limitations from artifacts related to cardiorespiratory motion and blood flow.3 By making repeated acquisition in each slice position to capture different phases of the cardiac- and respiratory cycles it is possible to largely circumvent these problems. The purpose of this study was to develop and evaluate improved image quality using a continuous Golden Angle radial acquisition scheme compared to Cartesian acquisitions with the same temporal footprint. By performing a continuous Golden Angle acquisition with the same acquisition time as the repeated Cartesian acquisitions, a sliding window reconstruction could offer better image quality with less motion- and flow artifacts while allowing greater freedom in choosing the temporal resolution for each slice position.Methods
Healthy volunteers with no known history of pulmonary disease were recruited to undergo MRI (n=10, age 46±11 years, 50% male). Images were acquired at 1.5 T (Siemens Area), using 34 surface coil elements. Parameters: flip angle, 60°; field-of-view, 450×450 mm2; matrix size, 288×288; voxel size, 0.6×0.6×3 mm3; slice thickness, 3 mm; 70 slices in the transversal plane. Images were acquired in two series using a balanced steady state free precession pulse sequence; once with a Cartesian trajectory (GRAPPA R=2, TE/TR: 1.6/3.2 ms, duration of image acquisition per slice: 584 ms) and once with a Golden Angle radial trajectory (144 radial spokes, TE/TR: 1.8/3.6 ms, duration of image acquisition per slice: 518 ms). Both acquisitions were made under free breathing with no further instructions given to the participants. The Golden Angle acquisition was reconstructed using SPIRiT4 (9×9 kernel). An intensity normalization map was estimated through adaptive coil combination5,6 and applied to the reconstructed images. The reconstruction was performed in MATLAB while the Cartesian data was reconstructed using the default settings on the scanner. The studies were anonymized and randomly mixed before being reviewed by two experienced radiologists. The image quality was scored on a scale from 1 (worst) to 5 (best) with respect to diagnostic quality, artifact level and vessel sharpness of the pulmonary vessels in the mediastinum, hilum, and lobar-, segmental- and subsegmental levels. In total, each study was scored for 63 different criteria using the ViewDEX software package.7 Observer scores were compared with Wilcoxon rank sum test. Visual Grading Characteristics (VGC) analysis, which is a non-parametric rank-invariant method for assessment of image quality from observer scores,8,9 was performed in VGC Analyzer.10,11 By calculating an image criteria score (ICS) for the test condition (Golden Angle) and the reference condition (Cartesian), an ROC-like curve of the proportion of the two ICS’s for various thresholds can be constructed. The area under the VGC curve gives a measure of how the two methods compare with respect to the specific criteria and observers.Results
The Golden Angle acquisition exhibited a higher observer score compared to the Cartesian acquisition (mean±SD, 2.52±0.87 vs 2.42±0.84, p<0.01), see Figures 1 and 2. Also, the area under the VGC curve comparing Golden Angle to Cartesian was 0.53 (95% CI 0.51 – 0.55, p = 0.006) indicating better image quality for Golden Angle.Discussion
Continuous Golden Angle radial sampling resulted in an improved image quality for pulmonary artery imaging compared to Cartesian sampling. Furthermore, the Golden Angle trajectory allows for a sliding window reconstruction with a large freedom when it comes to choosing a temporal resolution which may be shorter than the temporal footprint of the Cartesian acquisition.Conclusions
The Golden Angle acquisition had a better image quality compared to Cartesian acquisition. A continuous free breathing Golden Angle radial acquisition using balanced steady state free precession has the potential to improve visualization of pulmonary arteries with fewer artifacts. Further work will investigate the clinical ability of the proposed technique to detect acute pulmonary embolism in patientsAcknowledgements
No acknowledgement found.References
1. Goldhaber SZ, Bounameaux H. Pulmonary Embolism and Deep Vein Thrombosis. Lancet. 2012;379(9828):1835-1846.
2. Stein PD, Fowler SE, Goodman LR, et al. Multidetector Computed Tomography for Acute Pulmonary Embolism. N Engl J Med. 2006;354(22):2317-2327.
3. Nyrén S, Nordgren Rogberg A, Vargas Paris R, Bengtsson B, Westerlund E, Lindholm P. Detection of Pulmonary Embolism using Repeated MRI Acquisitions Without Respiratory Gating: A Preliminary Study. Acta Radiol. Preprint.
4. Lustig M, Pauly JM. SPIRiT: Iterative Self-Consistent Parallel Imaging Reconstruction from Arbitrary k-space. Magn Reson Med. 2010;64(2):457-471.
5. Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn Reson Med. 2000;43(5):682-690.
6. Griswold M, Walsh D, Heidemann RM, Haase A, Jakob P. The Use of an Adaptive Reconstruction for Array Coil Sensitivity Mapping and Intensity Normalization. Proc 10th Meet Int Soc Magn Reson Med. 2002
7. Håkansson M, Svensson S, Zachrisson S, Svalkvist A, Båth M, Månsson LG. ViewDEX: An Efficient and Easy-To-Use Software for Observer Performance Studies. Radiat Prot Dosimetry. 2010;139(1):42-51.
8. Båth M, Månsson LG. Visual grading characteristics (VGC) analysis: A Non-Parametric Rank-Invariant Statistical Method for Image Quality Evaluation. Br J Radiol. 2007;80(951):169-176.
9. Båth M. Evaluating Imaging Systems: Practical Applications. Radiat Prot Dosimetry. 2010;139(1):26-36.
10. Båth M, Hansson J. VGC Analyzer: A Software for Statistical Analysis of Fully Crossed Multiple-Reader Multiple-Case Visual Grading Characteristics Studies. Radiat Prot Dosimetry. 2016;169(1):46–53.
11. Hansson J, Månsson LG, Båth M. The Validity of using ROC Software for Analyzing Visual Grading Characteristics Data: An Investigation Based on the Novel Software VGC Analyzer. Radiat Prot Dosimetry. 2016;169(1):54-59.
Figures