3965
Optimized Trajectory Dual-Echo Fat and Water Separation1Department of Radiology, University of Wisconsin, Madison, WI, United States
Synopsis
Two-point Dixon methods enable robust fat suppression particularly in body and abdominal applications where large field of view imaging is required. However, a limitation of this approach is the decreased efficiency and longer TR’s necessary to acquire multiple echoes. Furthermore, due to system performance constraints, flexible choice of image parameters necessary to obtain near in and out-of-phase echo times limits valid combinations of field of view, matrix size, and encoding bandwidth. In this study, we describe a novel, generalized framework to optimize a ramp-sampled readout, which allows reduced scan time, flexible parameter selection, and improved quantitative fat and water separation.
Purpose
Reliable fat suppression over a large field of view is a particular challenge in MRI. At higher field strengths such as 3T, the desirable increased chemical shift between water and fat is complicated by B0 inhomogeneities. Recently, two-point Dixon methods1 have become popular to enable robust fat suppression particularly in body and abdominal applications. However, one of the limitations of the two-point Dixon approach is the decreased efficiency and longer TR’s necessary to acquire multiple echoes per k-space phase encoding. Furthermore, due to system performance constraints, flexible choice of image parameters necessary to obtain near in and out-of-phase echo times limits the valid combinations of field of view, matrix size, and encoding bandwidth. To ameliorate some of these issues, more flexible acquisitions using ramp sampling have been proposed2. In this study, we describe a novel, generalized framework to optimize the ramped readout. Using improved RF pulse design and a novel gradient calibration technique, we demonstrate imaging with reduced scan time, flexible parameter selection, and improved quantitative fat and water separation.Methods
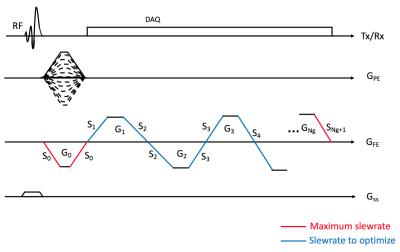
In this study we focus on ramp sampling based on a bipolar 3D gradient echo sequence as shown in Figure 1, where a SLR half pulse was utilized to shorten the sequence TR. The goal is to optimize the frequency encoding gradient, GFE, for ramp sampling with the following aspects: 1. Minimize the length of sequence, tseq. 2. Realize echoes at desired TEs within an acceptable error range, TEe. 3. Maximize readout duration to increase SNR. The parameters to optimize are slewrates, Sk (k=1,2,..,Ng, Ng=# of readout gradients), illustrated as blue lines in Figure 1. S0 and SNg+1 are fixed to a hardware (or patient) maximum slewrate, SHW. Readout gradients, Gk, are designed with constraints such that the area is equal to $$$2\pi N/(\gamma FOV)$$$, and its maximum amplitude is smaller than $$$2\pi/(\gamma FOVT_s)$$$, ( $$$\gamma$$$: a gyromagnetic ratio, FOV: field of view, N: matrix size, Ts : sampling time interval).
The targeted parameters, Sk (k=1,...,Ng), are optimized based on the following cost function.
$$C = t_{seq} + \sum_{k=1}^{Ng}(\lambda_kC_{TE}(\triangle TE_k,TE_e)+\mu_kS_k) $$
, where ΔTEk is error between desired TE and realized TE at k-th echo, and λk and μk are weighting factors. CTE is defined as a piecewise function as $$$C_{TE} (x,x_0 )=|x|\ if\ x<x_0,\ x^2\ if\ x≥x_0.$$$
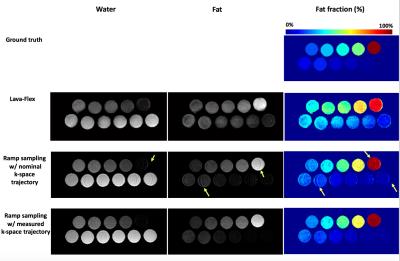
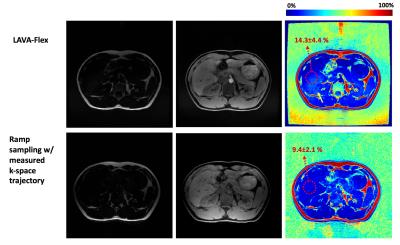
The best set of slewrates can be determined by iteratively minimizing the given cost function. In each iteration, hypothetical readout gradients are designed based on the updated slewrates, and the resultant cost is calculated. In this study, Nelder-Mead simplex (fminsearch in MATLAB) was used to optimize with initial guesses set to be SHW/2. To evaluate the proposed method, readout gradients for two-point Dixon was optimized with the parameters: FA=10o, targeted TE=1.1ms/2.2ms, TEe=100µs, λk=μk=10, SHW=118mT/m/ms, matrix size=288x192x50, FOV=380x380x300mm3. The optimized ramp sampling was compared with a clinically available two-point Dixon sequence (LAVA-Flex) on 3T MR scanner (Signa PET/MR, GE Healthcare, Waukesha, WI). 40-channel anterior and posterior array coils were used for imaging. In the phantom experiment, 11 vials with 0, 2.5, 5, 7.5, 10, 15, 20, 30, 40, 50, and 100% fat were imaged. For the In vivo experiment, a breath-held abdominal imaging (using 2x ARC parallel imaging) was performed in a human subject. For ramp sampling, images were reconstructed using convolution gridding, and k-space trajectory measured using SPI-based calibration3. Fat and water separation and ARC reconstruction was performed using the GE Healthcare Orchestra SDK (v1.44.722).
Results and Discussion
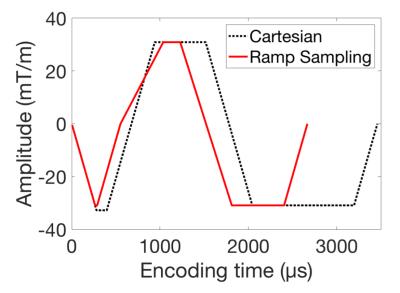
Figure 2 shows the conventional Cartesian sampling and optimized readout gradients for ramp sampling. For ramp sampling, the optimized parameter S1 was 63.9mT/m/ms and S2 was 105.8mT/m/ms with realized TE1=1084µs and TE2=2100µs. The resultant tseq was 2668µs which is 23% reduction compared with tseq=3460µs in the corresponding Cartesian sampling. Total scantime for optimized ramp sampling and LAVA-Flex was respectively 30sec and 39sec in phantom experiment, and respectively 19sec and 22sec with parallel imaging in vivo experiment. Figure 3 shows the estimated fat fraction from the phantom experiment, where the optimized ramp sampling with the measured k-space trajectory shows the best fat fraction estimates. Figure 4 shows fat/water/fat fraction estimated from the vivo experiment, where ramp sampling shows improved results compared to the standard LAVA-Flex acquisition which shows artifactual biasing in liver fat fraction as the yellow arrow indicates.Conclusion
In this study, we have presented a new framework to optimize readout parameters for ramp sampling. The use of an optimized readout reduced scan time, and k-space trajectory measurement allowed an improved assessment of quantitative fat fraction compared to the standard clinical sequence.Acknowledgements
We acknowledge support from NIH EB013770 and GE Healthcare.References
1. Li XH, Zhu J, Zhang XM, Ji YF, Chen TW, Huang XH, Yang L, Zeng NL. Abdominal MRI at 3.0 T: LAVA-Flex compared with conventional fat suppression T1-weighted images. J. Magn. Reson. Imaging 2014;40:58–66. doi: 10.1002/jmri.24329.
2. Hwang K-P, Rambow O, Bayram E, Hazl JD, Madewell JE, Slavens ZW, Vu AT, Ma and J. Improved single-pass dual echo Dixon imaging with ramp sampling and flexible echo times. In: Proc Intl Soc Mag Reson Med. Vol. 21. ; 2013. p. 2406.
3. Jang H, McMillan AB. A rapid and robust gradient measurement technique using dynamic single-point imaging. Magn. Reson. Med. 2016;00. doi: 10.1002/mrm.26481.
Figures