3961
Fast T1 Correction for Fat Quantification using a Dual-TR Chemical Shift Encoded MRI Acquisition1Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 4Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States, 5Medicine, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Dual flip angle (DFA) methods facilitate T1-corrected proton density fat-fraction (PDFF) quantification at the cost of doubling scan time compared to small flip angle (SFA) methods. In this study, a novel “dual-TR” (DTR) fat quantification strategy was proposed. It acquired 2 spoiled gradient echo (SGRE) dataset sequentially, one with a shortened echo train and reduced TR to alleviate scan time penalties. Monte-Carlo simulation and Cramer-Rao lower bound demonstrated improved noise performance using the proposed method compared with SFA and DFA methods. Phantom experiments demonstrated the feasibility of T1-corrected PDFF estimates using the proposed DTR method.
Introduction
In chemical-shift encoded (CSE) quantification of proton density fat-fraction (PDFF), a multi-echo spoiled gradient echo (SGRE) acquisition with a small flip angle (SFA) is typically used to avoid T1-related bias1,2,3,4. However, SFA acquisitions result in low SNR. Alternatively, a dual flip angle (DFA) approach enables T1-corrected PDFF estimates4. Unfortunately, DFA methods double the scan time, which is problematic for breath-held abdominal applications. The purpose of this work is to propose and investigate the feasibility of a novel “dual-TR” (DTR) T1-corrected fat quantification method based on 2 sequentially acquired SGRE acquisitions, one of which has a shortened echo train and shorter TR to minimize the scan time penalty.Materials and Methods
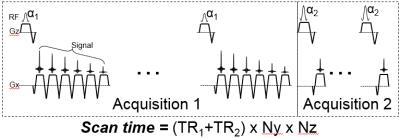
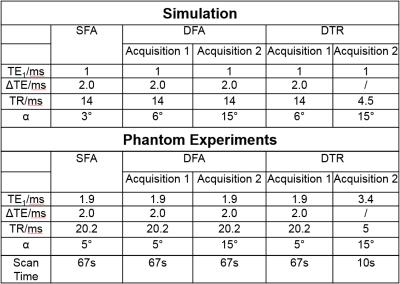
The proposed DTR acquisition is described in Figure 1 where a multi-echo CSE-MRI acquisition is immediately followed by a second acquisition with a reduced echo train and a shorter TR.
Signal model, acquisition and reconstruction: A generalized single-voxel signal model of a multi-echo SGRE acquisition, with different T1-weightings (through changes in TR and/or flip angle) can be written:
$$s(W,F,{\phi},R2^{*},{\psi},T1_{w},T1_{f};t_{n,m},\alpha_{m},TR_{m})=e^{i(2{\pi}{\phi}t_{n,m})}e^{-R2^{*}t_{n,m}}e^{i\psi}(W\frac{(1-e^{\frac{-TR_{m}}{T1_{W}}})sin({\alpha})}{(1-e^{\frac{-TR_{m}}{T1_{W}}}cos({\alpha}))}+F\frac{(1-e^{\frac{-TR_{m}}{T1_{F}}})sin({\alpha})}{(1-e^{\frac{-TR_{m}}{T1_{F}}}cos({\alpha}))})$$
Where W and F are the amplitudes of water and fat proton densities, ψ is the common initial phase of water and fat signal. φ denotes the field inhomogeneity of the voxel (Hz). TRm, αm are repetition time and flip angle of the mth acquisition. tn,m denotes the echo time of the nth echo in the mth acquisition. The proposed technique estimates PDFF by performing a non-linear least-squares estimation of the proton densities of water (W) and fat (F) and thus T1-corrected estimation of PDFF. Importantly, a useful byproduct of this approach is that the T1 of both water and fat components are also estimated.
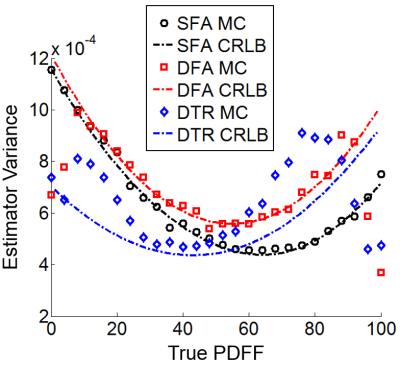
Simulation: Monte-Carlo simulations based on SFA, DFA and DTR methods were performed to assess the noise performance of the corresponding PDFF estimators. 3000 trials were performed over a PDFF range of 0-100%. The signal was generated using the following parameters5,6: T1w=583m, T1f=343ms, R2*=40s-1, and B0=1.5T. Cramer-Rao lower bound (CRLB) of the PDFF estimates for each technique (SFA, DFA and DTR) was calculated using the same parameters for comparison.
Acquisition parameters (Table 1) were chosen within reasonable ranges to minimize the maximum estimator variance predicted by CRLB over PDFF between 0% to 40% (the range typically seen in liver disease). A flip angle of 3° was chosen for the SFA based on recent empirical studies7. White Gaussian noise was chosen such that SNR is approximately 20 for SFA acquisition. SNR was normalized by square root of scan time in DFA and DTR. T1w, T1f were constrained to [1ms, 2000ms] in non-linear least-squares fit4 in DFA and DTR to avoid instability when water or fat signal was low.
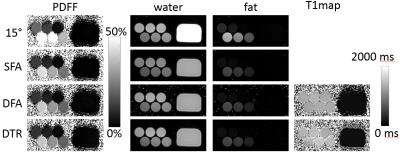
Phantom Experiments: Phantom experiments were performed on a clinical 1.5T system (HDxt, GE Healthcare) using a single-channel quadrature head coil to evaluate the feasibility of the proposed DTR method. SFA, DFA and DTR fat quantification with parameters listed in Table 1 were performed on a PDFF phantom consisting of multiple vials with PDFF value of 0%,5%,10%,20%,30%,40%, respectively, and T1water≈1400ms, T1fat≈300ms. A doped water phantom with T1=110ms was added for validation of T1 estimates. In addition, a single SGRE acquisition with α =15° was acquired to demonstrate the T1-bias that occurs when a single large flip angle is acquired.
Results:
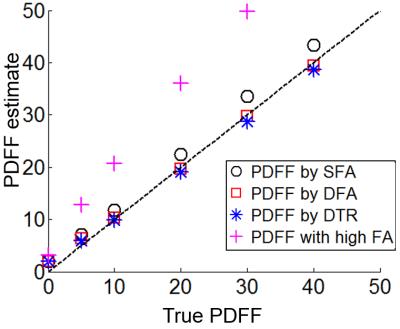
CRLB and Monte-Carlo simulation showed good agreement and improved noise performance of the DTR method compared with SFA and DFA (Figure 2). The agreement between CRLB and Monte Carlo simulations breaks down for very low and very high fat fraction due to the difficulty in estimating T1 with low signals from the minority species and the constrains imposed on the least square fit. The proposed method provided T1 corrected fat quantification (Figure 3). SFA, DFA and DTR methods all successfully reduced T1 bias. SFA showed some residual T1 bias while DFA and DTR further reduced T1 bias in their PDFF estimates. This comparison of residual T1 bias was further quantitatively confirmed by the PDFF estimates averaged inside an ROI for each method (Figure 4).Discussion:
The proposed DTR method provides a fully T1 corrected estimation of PDFF. Further, we have demonstrated improved theoretical noise performance of DTR compared with SFA and DFA methods. The reduced scan time of the DTR method, compared with DFA approaches, makes it more practical for abdominal applications such as fat quantification in the liver. The proposed method is a promising technique for accurate T1 corrected fat quantification in the abdomen. Further work is needed to implement and validate these methods in vivo.Acknowledgements
The authors wish to acknowledge support from the NIH (R01 DK083380, R01 DK088925, R01 DK100651, K24 DK102595), as well as GE Healthcare.References
[1] Meisamy, et al, Radiology 2011; 258:767-775.
[2] Hines, et al, JMRI 2011;33:873-881.
[3] Hines, et al, Radiology 2011;258:767-775
[4] Liu, et al, MRM 2007;58:354-364.
[5] de Bazelaire, et al, Radiology 2004;230:652-659.
[6] Westwoord, et al, JMRI 2003;18:33-39.
[7] Johnson, et al, JMRI 2014; 39; 440-447.
Figures