3946
A novel and robust reconstruction method for free-breathing cine DENSE by minimizing k-space entropy1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Medicine, University of Virginia, Charlottesville, VA, United States, 3Radiology, University of Virginia
Synopsis
A reliable free-breathing (FB) cine DENSE method would benefit myocardial strain imaging in many patients. An echo due to T1 relaxation is an important source of artifacts, particularly for free-breathing acquisitions. We propose to optimize suppression of these echoes by minimizing k-space entropy. The method was tested in 10 subjects (6 healthy volunteers and 4 heart failure patients) and compared to a conventional diaphragm navigator method (dNAV). Image reconstruction by minimizing k-space entropy provided better image quality than the conventional dNAV method.
Background
Cine DENSE is an accurate myocardial strain imaging technique that typically employs breath-holding acquisition.1 A reliable free-breathing (FB) method would be beneficial for many patients, such as heart failure and pediatric patients. Previous work has utilized diaphragm-based navigators (dNAVs)2 and image-based self-navigation (iNAV)3 methods for this purpose with moderate success. In particular for DENSE, sufficient suppression of the artifact-generating echo due to T1 relaxation is vital, where suppression of this echo is generally achieved by subtraction of complementary phase-cycled datasets.1,4 However, for free-breathing acquisitions, cancellation of this echo is compromised when phase-cycled datasets are acquired at different respiratory positions. We have observed that poor suppression of the T1 relaxation echo due to respiration results in a greater spread of energy in k-space, which can be measured as k-space entropy. In this work, we propose to optimize suppression of the T1 relaxation echo and compensate for respiratory artifacts in cine DENSE by minimizing k-space entropy.Methods
Rationale: In cine DENSE, T1 relaxation echoes co-exist with stimulated echoes (STE) and generate artifacts. T1 relaxation echoes grow with time in the heart-cycle and arise from the entire slice, whereas STE do not grow and can be localized to a focused region using slice-selective preparation RF pulses. We propose a strategy for free-breathing cine DENSE reconstruction to identify phase-cycling pairs that have sufficient suppression of T1 relaxation echoes.
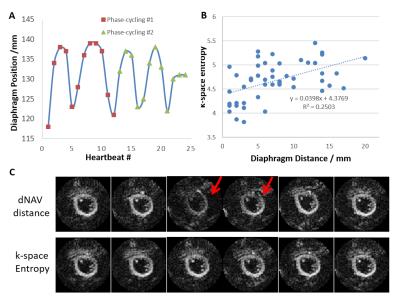
The concept of entropy has been extended to measure histogram flatness and sharpness of a grayscale image.5, 6 DENSE images with sufficient suppression of T1 relaxation signals have a single peak at the k-space center, while images with insufficient T1 echo suppression have strong signals around the displacement-encoding frequency as well,4 which results in a flatter histogram of the k-space magnitude, as show in Figure 1 (A-D). We define the entropy of the k-space magnitude as k-space entropy to quantify the sufficiency of T1 relaxation echo suppression (Figure 1 (E)) and to identify phase-cycling pairs with minimal k-space entropy after phase-cycling subtraction.
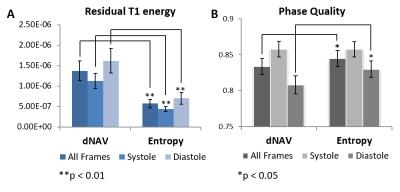
Data acquisition and reconstruction: 2D free-breathing DENSE data were acquired in 6 healthy volunteers and 4 heart failure patients using spiral acquisition and localized STE methods (3 orthogonal slice-selective RF pulses) on 3T systems (Trio and Skyra, Siemens). A short axis mid-ventricular slice was imaged with the following parameters: field of view of 160 mm, 6 interleaves per image, 2 interleaves per heartbeat, temporal resolution of 30 ms, 4 averages, and total scan time of 72 heartbeats. dNAVs were acquired at the end of each heartbeat. Each dataset was reconstructed two ways: accept heartbeats that are within the diaphragm acceptance window (+/- 3 mm at expiration) and accept data that minimizes k-space entropy after phase-cycling subtraction. The residual T1 relaxation echo energy and phase quality in the myocardial region-of-interest were calculated to quantify the resulting image quality.
Results
Figure 2 shows an example of data selection by dNAV and k-space entropy. Respiratory motion tracked by dNAV positions is shown in figure 2 (A). The k-space entropy of all phase-cycling pairs positively correlated with the correspding dNAV distances. Images corresponding to the first 6 phase-cycling pairs with minimal dNAV distances or minimal k-space entropy are shown in figure 2 (C). 2 out of the 6 phase-cycling pairs by dNAV have poor quality (red arrow) while all 6 pairs by k-space entropy have good quality. This example demonstrates that k-space entropy identifies phase-cycling pairs with successful T1 echo subtraction more accurately than dNAV.
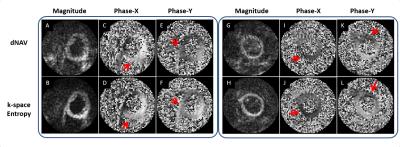
Figure 3 shows diastolic DENSE images reconstructed from two examples. For both examples, the reconstructions by dNAV have strong striping and aliasing artifacts in magnitude and phase images (red arrows); while the minimum-entropy method shows much better image quality with higher signal-to-noise ratio and less phase error. Image quality for data from all subjects is summarized in Figure 4. Residual T1 relaxation energy by the minimum-entropy method is significantly lower than dNAV (p<0.01). Phase quality by the minimum-entropy method is significantly higher than dNAV (Diastole, 0.83 +/- 0.01 vs. 0.81 +/- 0.01, p<0.05).
Conclusion and Discussion
The k-space entropy based method is a novel and robust method for free-breathing cine DENSE imaging. The method provides better suppression of T1 relaxation echoes than conventional dNAV and higher quality DENSE image reconstruction. The method is purely data-driven and does not require knowledge of location of the T1 relaxation echoes in k-space. It can also be adapted for 3D imaging easily. Future work will develop motion correction after entropy-based phase-cycling subtraction and methods to increase efficiency by adaptively determining an accept/reject threshold of the k-space entropy.Acknowledgements
This work was supported by NIH R01EB001763 and an award from the American Heart Association and the Children’s Heart Foundation, AHA16PRE30680001.References
1. Kim, Daniel, et al. “Myocardial Tissue Tracking with Two-dimensional Cine Displacement-encoded MR Imaging: Development and Initial Evaluation.” Radiology 230.3 (2004): 862-871.
2. Zhong, Xiaodong, et al. “Imaging three-dimensional myocardial mechanics using navigator-gated volumetric spiral cine DENSE MRI.” Magnetic resonance in medicine 64.4 (2010): 1089-1097.
3. Cai, Xiaoying, et al. “Free-breathing 2D Cine DENSE with localized excitation, self-navigation and motion correction”. Journal of Cardiaovascular Magnetic Resonance 2016, 18(Suppl 1):P319
4. Epstein, Frederick H., and Wesley D. Gilson. “Displacement-encoded cardiac MRI using cosine and sine modulation to eliminate (CANSEL) artifact-generating echoes." Magnetic resonance in medicine 52.4 (2004): 774-781.
5. Shannon, Claude Elwood. "A mathematical theory of communication." ACM SIGMOBILE Mobile Computing and Communications Review 5.1 (2001): 3-55.
6. Kapur, Jagat Narain, Prasanna K. Sahoo, and Andrew KC Wong. "A new method for gray-level picture thresholding using the entropy of the histogram." Computer vision, graphics, and image processing 29.3 (1985): 273-285.
Figures