3945
Regularly Incremented Phase Encoding Magnetic Resonance Fingerprinting (RIPE-MRF) for Enhanced Motion Suppression in Cartesian MRF1Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States, 2Radiology, University Hospitals Cleveland Medical Center, Cleveland, OH, United States, 3Physiology and Biophysics, Case Western Reserve University, Cleveland, OH, United States, 4Pediatrics, University Hospitals Cleveland Medical Center, Cleveland, OH, United States
Synopsis
Preclinical quantitative MRI is susceptible to the motion artifacts caused by the rapid respiratory motion and high heart rates present in small animals. This can be alleviated through the use of gating/triggering but these are difficult to implement in magnetic resonance fingerprinting due to the need for dynamic, coherent signal evolutions. We propose a method for an incremented phase encoding MRF acquisition that enhances motion suppression in Cartesian MRF. This phase incremented strategy distributes motion artifacts throughout the acquisition creating incoherent artifacts allowing the MRF method to “see through” the artifacts and produce artifact free T1 and T2 maps.
Purpose
The presence of motion artifacts is of significant concern for preclinical MRI due to the lack of breathholds and rapid breathing and heart rates in animal models. These motion artifacts are typically limited with respiratory and/or cardiac gating1. Unfortunately, gating in MRF is challenging as MRF requires dynamic, coherent signal acquisitions. Prior preclinical studies have shown that Cartesian MRF exhibits some inherent resistance to respiratory motion artifacts. However, these in vivo studies have also shown that Cartesian MRF is still subject to major pulsatility artifacts as well as residual respiratory motion artifacts2. In this study, we propose to implement a Cartesian MRF acquisition with incremented phase encoding3,4 during each dynamic MRF acquisition to introduce an additional temporal incoherence in order to “dephase” the motion and pulsatility artifacts.Methods
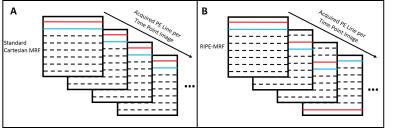
Two fully-sampled Cartesian FISP MRF5 acquisitions were implemented on a high field (7T) Bruker small animal MRI scanner. The first acquisition was a standard Cartesian MRF implementation with a constant phase encoding for each image of the 1024 images in the dynamic MRF scan as described previously2. The second scan, termed Regularly Incremented Phase Encoding MRF (RIPE-MRF), incorporated a time-varying incremented phase encoding step across all of the 1024 images. These are visually demonstrated in Figure 1. The repetition time (9.5-12 ms) and flip angle (0-70 degrees) profiles were the same for both MRF acquisitions and similar to previous MRF implementations6. We first obtained in vitro phantom MRF results to ensure the consistency between the two MRF methods. We then obtained in vivo MRF data of mouse kidneys to qualitatively compare the motion artifact sensitivity for both the standard Cartesian MRF and RIPE-MRF methods. The MRF data were acquired for the same imaging slice and with no respiratory or cardiac gating. All MRF datasets were matched on a pixel-by-pixel basis using the same MRF dictionary to generate T1 and T2 maps.Results
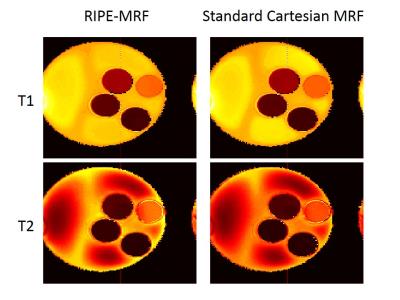
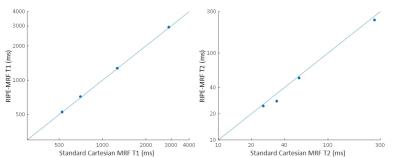
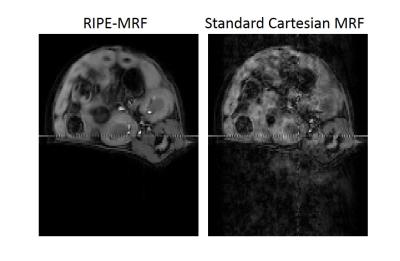
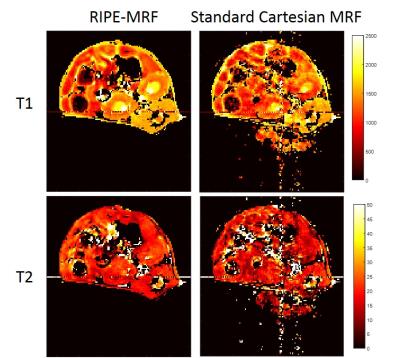
The in vitro MRF results from the standard Cartesian MRF and RIPE-MRF techniques are shown in Figures 2 and 3. These two MRF methodologies generated similar T1 and T2 maps (Figure 2) with consistent T1 and T2 values between RIPE-MRF and standard Cartesian MRF (Figure 3). In vivo MRF results for the two MRF methods are shown in Figures 4 and 5. Composite MRF images were calculated by performing a complex sum of the 1024 images for each MRF acquisition (Figure 4). Interestingly, the composite image from the RIPE-MRF acquisition shows significantly reduced artifact in comparison to the composite image from the standard Cartesian MRF acquisition. From ROI analysis the artifact-to-noise ratio in the composite images from standard Cartesian MRF was measured to be 12.758 and for the RIPE-MRF composite image the artifact-to-noise ratio was 1.620. Similar reduction in motion and pulsatility artifact were observed in the in vivo T1 and T2 maps (Figure 5).Discussion
In this study, we have shown that incorporation of incremental phase encoding during the dynamic MRF scan (RIPE-MRF) results in temporal incoherence of the pulsatility and motion artifacts. By incrementing the phase encoding lines of k-space, RIPE-MRF distributes the occurrence of any motion artifacts and any variation in physiological state over the entire acquisition rather than a single line of k-space. This temporal incoherence resulted in a significant reduction in the level of artifact in both the composite images (Figure 4) as well as the corresponding T1 and T2 maps (Figure 5). In vitro results suggest that this reduction in motion/pulsatility artifact does not significantly impact the accuracy of the T1 and T2 estimates (Figures 2 and 3).Conclusion
The proposed RIPE-MRF technique offers the opportunity to improve the quality of Cartesian MRF results for imaging applications where motion and cardiac pulsatility are problematic. The RIPE-MRF technique allows Cartesian MRF acquisitions to be implemented without respiratory or cardiac gating allowing for a more rapid acquisition with reduced complexity and post-processing.Acknowledgements
NIH T32EB007509, NIH TL1TR000441, NHLBI R21 HL130839, Cystic Fibrosis FoundationReferences
1. Zhang H, Ye Q, Zheng J, et al. Improve myocardial T1 measurement in rats with a new regression model: application to myocardial infarction and beyond. Mag Res Med. 2014. 72: 737-748.
2. Gao Y, Chen Y, Ma D, et al. Preclinical MR fingerprinting (MRF) at 7T: effective quantitative imaging for rodent disease models. NMR Biomed. 2015; 28: 384-394.
3. Liu G, Sobering G, Duyn J, et al. A functional MRI technique combining the principles of echo-shifting with a train of observations (PRESTO). Mag Res Med. 1993. 30(6): 764-768.
4. Oshio K, Feinberg D. GRASE (Gradient-and Spin Echo) imaging: A novel fast MRI technique. Mag Res Med. 1991. 20(2): 344-349.
5. Jiang Y, Ma D, Seiberlich N, et al. MR Fingerprinting using fast imaging with steady state precession (FISP) with spiral readout. Mag Res Med. 2015. 74(6): 1621-1631.
6. Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. 2013. Nature. 495: 187-192.
Figures