3941
Real-time prospective bulk motion exclusion for robust 3D free-breathing abdominal imaging1Department of Radiotherapy, University Medical Center Utrecht, Utrecht, Netherlands, 2Center of Advanced Imaging Innovation and Research (CAI2R), Department of Radiology, New York University School of Medicine, New York, NY, United States, 3Bernard and Irene Schwartz Center for Biomedical Imaging, Department of Radiology, New York University School of Medicine, New York, NY, United States
Synopsis
3D free-breathing approaches have shown potential for abdominal imaging in patients who are unable performing breath-holds, such as pediatric patients and uncooperative adults. However, these free-breathing techniques fail when bulk motion occurs. This study proposes a method to detect and exclude such bulk motion to ensure diagnostic image quality, even in this challenging group of patients. Without requiring user interaction, this technique improves robustness for abdominal MR imaging, while minimizing the scan time on an individual basis, using a real-time implementation on the MRI system, which may enable robust non-sedated pediatric imaging.
Purpose
Free-breathing approaches have shown great promise for 3D abdominal imaging in patients who have difficulties performing breath-holds1,2,. However, these techniques fail when non respiratory-related bulk movements occur3. Sudden motion events, such as a global body shifts, bulk motion, or coughing may induce motion artifacts, resulting in images of non-diagnostic quality. This is especially problematic when imaging pediatric patients and children without use of anesthesia, but also in uncooperative or elderly adults. The goal of this study was to develop a data-consistency-driven image stabilization technique, which detects and excludes bulk movements from the acquired k-space data without any user interactions.Methods
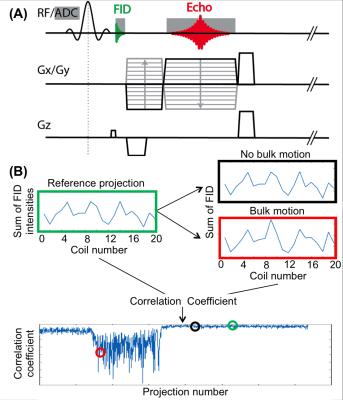
Bulk motion detection: A 3D radial stack-of-stars acquisition was modified to acquire a free induction decay (FID) signal prior to each read-out line (Figure 1a)4. These signals were processed in real time on the scanner. To reduce latency, only the FID samples of the center k-space partition were sent to the real-time processing unit. A time series of 32 FID samples were acquired and summed, resulting in one value per projection for every receive channel, which were then concatenated into a vector (Figure 1b)5. Correlation coefficients were calculated between a reference projection and all other projections. A low correlation coefficient implies that the load distribution of the receive-coil elements has changed. Since all coil elements have varying sensitivity, this indicates that the patient has moved. Since it is unknown beforehand if and when bulk motion occurs, the reference projection was dynamically updated, based on the highest overall correlation with all previous projections. To determine outlier projections, which indicate bulk motion, soft-thresholding with a user-defined acceptance threshold was employed. Once a user-defined number of consecutive, accepted projections was acquired, the scan was considered successful and the acquisition was automatically stopped. If this consistency window could not be detected within a predefined maximal scan time, the scan was terminated and the longest window of consecutive projections was identified and used for image reconstruction.
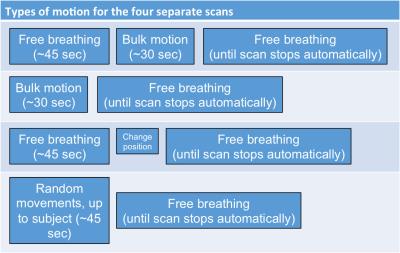
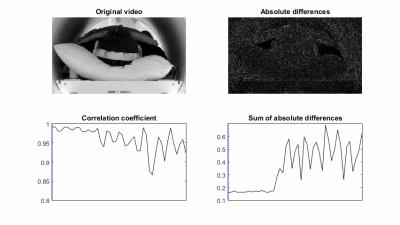
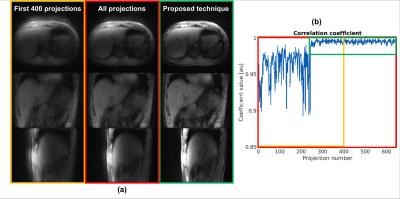
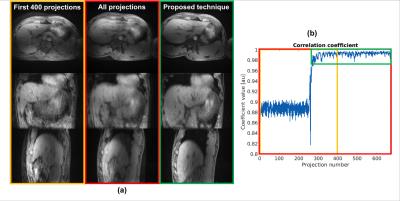
Data acquisition and evaluation: Five healthy volunteers were scanned on a 3T scanner (Skyra, Siemens Healthineers) using the modified 3D radial stack-of-stars, fat-suppressed, spoiled gradient-echo protocol, based on the golden angle scheme6 (FOV = 350x350x216 mm3, voxel size = 1.36x1.36x3.0 mm3, flip angle = 12o, TE/TR = 1.71/3.35 ms, bandwidth = 890 Hz/px). For each volunteer, four separate scans with different motion tasks were performed (Figure 2). The maximum scan time was set to 5m11s (corresponding to 1500 projections), while the length of the acceptance window was set to 400 projections. A threshold of 0.975 was used. After each scan, the accepted window borders and correlation coefficients, calculated by the real-time feedback system, were exported. To demonstrate the effect of the proposed image-stabilization technique, the automatically reconstructed images were compared with two retrospectively reconstructed data sets: 1) Only the first 400 projections were used for reconstruction, corresponding to the same window length, and 2) all acquired projections were used for reconstruction, corresponding to the same maximum scan duration. For validation of the detected motion signal, one subject was video recorded (Powershot SX170IS, Canon Inc, Tokyo, Japan). Motion in this video was extracted by calculating the sum of the absolute differences on a frame-by-frame basis.
Results
Figure 3 shows a snapshot of the video when the subject started to move severely. This motion can be seen as an increase in the absolute differences, which correlates well with a decrease in correlation coefficients. Figure 4 displays the reconstructed volumes, along with the correlation coefficients and accepted windows for one subject that moved during the first ~45 secs. A clear increase in image sharpness and reduction of streaking artifacts is observed in all planes. Figure 5 shows results for another subject that changed position once. A major decrease of shine-through and increase of image quality can be seen, primarily in the axial and sagittal view.Discussion and Conclusion
Despite novel techniques for motion-robust imaging, occurrence of bulk motion can still result in non-diagnostic image quality. This work demonstrates an automatic framework to detect and exclude undesirable non-physiological motion from continuously acquired abdominal acquisitions. Residual motion artifacts that are present in the shown images are attributed to physiological respiratory motion, which intentionally is not identified by the proposed method. Respiratory motion can be subsequently reduced by using time-efficient techniques such as soft-gating7 or XD-GRASP8. Altogether, the presented technique improves robustness for abdominal MR imaging and may be of particular value for imaging pediatric patients without use of sedation.Acknowledgements
Part of this research was funded by NIH 5R01EB018308 and the Royal Netherlands Academy of Arts and Sciences (KNAW) Ter Meulen Grant.References
[1] Zhang T, et al. Fast pediatric 3D free-breathing abdominal dynamic contrast enhanced MRI with high spatiotemporal resolution. jMRI 2015;41:460-473
[2] Chandarana H, et al. Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: a viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Invest Radiol 2011;46:648-653
[3] Higano NS, et al. Retrospective respiratory self-gating and removal of bulk motion in pulmonary UTE MRI of neonates and adults, MRM 2016, Epub ahead of print, doi: 10.1002/mrm.26212
[4] Kober T, et al. Prospective and retrospective motion correction in diffusion magnetic resonance imaging of the human brain, NI 2012;59:389-398
[5] Hu P, et al. Motion Correction using coil arrays (MOCCA) for free-breathing cardiac cine MRI, MRM 2011;66:467-475
[6] Winkelmann S, et al. An optimal profile order based on the golden ratio for time-resolved MRI, IEEE TMI 2007;26:68-76
[7] Cheng JY, et al. Free-breathing pediatric imaging with nonrigid motion correction and parallel imaging, 2013, ISMRM 22, p312
[8] Feng L, et al. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing, MRM 2016;75:775-788
Figures