3934
A generalized prospective motion correction framework for improved spectroscopy, structural and angiographic imaging1Philips Healthcare, Copenhagen, Denmark, 2Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital Hvidovre, Denmark, 3Electrical Engineering, Technical University of Denmark, Lyngby, Denmark
Synopsis
Subject motion is a major problem in brain imaging and spectroscopy, misleading diagnosis in the clinic and lowering data quality in research. A promising solution is to update the field-of-view in real time based on tracking with volumetric navigators. This was implemented using a new interleaved scanning framework and a product functionality for self-navigation of fMRI. The implementation is simple to use and very flexible, as the navigator and target sequence are simply defined as two different scans, that can be interleaved at any level. It was demonstrated to improve motion robustness in single voxel spectroscopy, T1-weighted imaging and angiography.
Purpose
To exploit a recently developed interleaved scanning framework1 for a flexible implementation of prospective motion correction using volumetric navigators in brain imaging and spectroscopy.Methods
The interleaved scanning framework1 allows instantaneous switching between multiple sequences at any time point in the execution, using a generic function call, scan_handover( ). Therefore, the navigator sequence and target sequence (e.g. MPRAGE or spectroscopy) were simply defined as two different sequences.
In the navigator sequence, the prospective motion correction (PMC) functionality available on the scanner was turned on, which is designed for self-navigation of fMRI sequences. The PMC functionality registers the latest acquired dynamic volume to the first, and uses the realignment parameters for updating the translation offsets and orientation matrix of the fMRI sequence in real-time (similar to ref. 2 ). For the $$$n$$$‘th update of the navigator sequence (subscript $$$nav$$$), the corresponding geometry updates for the target sequence (subscript $$$ts$$$) were computed as :
$$O^n _{ts}=\Delta R O^{n-1} _{ts},$$
$$t^n_{ts}=\Delta R (t^{n-1}_{ts} -t^{n-1}_{nav})+t^{n}_{nav},$$
where
$$\Delta R = O^n _{nav} (O^{n-1} _{nav})^{-1},$$
and $$$O^n _{ts}, O^n _{nav}$$$ are the $$$n$$$'th orientation matrices (transferring from slice selection, phase encoding, readout directions to the physical coordinate system of the scanner); $$$t^{n}_{nav}, t^{n}_{nav}$$$ are the $$$n$$$'th translation offsets in the physical coordinate system of the scanner.
To demonstrate the flexibility, navigators were implemented for three different target sequences.
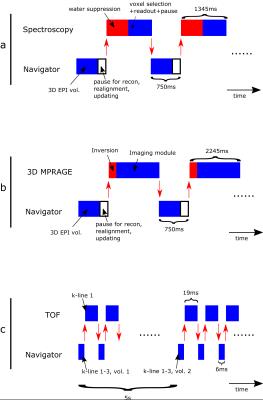
a) Single voxel spectroscopy (PRESS). A 3D EPI sequence (resolution 7x7x8 mm3, tip angle=2⁰, similar to the vNAV in ref. 3) was used as the navigator sequence, and one volume was fitted in the waiting time between each spectroscopy acquisition (Figure 1a).
b) 3D MPRAGE sequence. The same navigator sequence was used as for the spectroscopy, but here the scan_handover() was called such that one EPI volume was in the dead time between one imaging module and the next inversion pulse (Figure 1b).
c) Time of flight (TOF) sequence. For sequences with no magnetization preparation and a short TR, fitting in a whole EPI volume may be difficult (fitting it in for every shot prolongs the TR and scan time significantly, fitting it in only every N’th shot makes the steady state magnetization vary over the scan). Instead, the navigator sequence was defined as a low resolution 3D gradient echo sequence (resolution 7x7x8 mm3, tip angle=2⁰) and the scan_handover() calls were placed such that one shot of the TOF sequence was interleaved with 3 shots of the navigator (see Figure 1c).
The prospective correction of all three sequences were tested in healthy volunteers, by acquisitions with/without motion and prospective motion correction turned on/off. To be able to reproduce the motion across the scans with and without correction, the subject was asked to move once, half way through each scan, and hold that position for the rest of the scan. For the spectroscopy sequence, the voxel was placed close to the skull, so motion became apparent by spectrum contamination by lipids.
All experiments were performed on a 3 T MRI system (Achieva, Philips Healthcare, Best, Netherlands) equipped with a 32 channel receive head coil.
Results
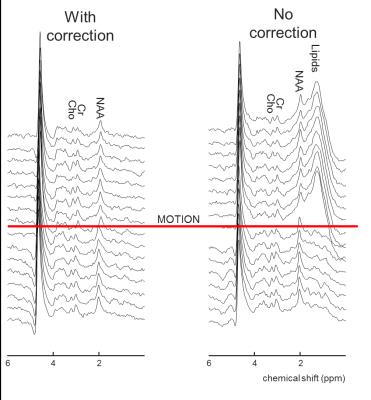
Figure 2 show results from the spectroscopy experiment. Without motion correction, all individual spectra after the motion occurred are contaminated by lipids, while that is not the case when the proposed prospective motion correction method is used.
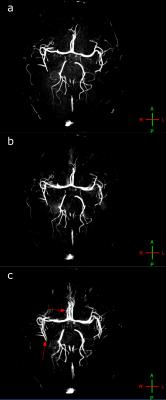
Figure 3 and 4 show results from the imaging experiments. With motion and no correction, severe artefacts occur with both scans – blurring is seen in the MPRAGE (Figure 3c), and duplication of some of the vessels are seen in the TOF (Figure 4c). When prospective corrections are applied, the image quality is significantly improved (Figure 3b and 4b). Without deliberate motion, the image quality is still the best (Figure 3a and 4a).
Discussion
The presented implementation makes it simple and flexible to add prospective motion correction with volumetric navigators to a sequence. The flexibility is demonstrated by the incorporation into the TOF sequence, where only part of the navigator is acquired between each shot in the host sequence. This is accomplished by just defining a proper scan in the normal user interface, and positioning the scan_handover() call in the execution list.
The fact that some artefacts remained in the images though prospective motion correction was performed is likely due to the corrupted data acquired after the motion occurred, before the next navigator volume was finished, and because motion was provoked when the acquisition was close to the center of k-space. This will be improved in the future by implementation of a reacquisition scheme.
Acknowledgements
This research is supported by the Danish Council for Independent Research [grant no. 6111-00349A].References
1. M. Henningsson, G. Mens, P. Koken, J. Smink, and R. M. Botnar, “A new framework for interleaved scanning in cardiovascular MR: Application to image-based respiratory motion correction in coronary MR angiography,” Magn. Reson. Med., vol. 73, no. 2, pp. 692–696, Feb. 2015.
2. S. Thesen, O. Heid, E. Mueller, and L. R. Schad, “Prospective acquisition correction for head motion with image-based tracking for real-time fMRI.,” Magn. Reson. Med., vol. 44, no. 3, pp. 457–65, Sep. 2000.
3. M. D. Tisdall, A. T. Hess, M. Reuter, E. M. Meintjes, B. Fischl, and A. J. W. Van Der Kouwe, “Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI,” Magn. Reson. Med., vol. 68, no. 2, pp. 389–399, 2012.
Figures