3931
A Registration Framework for Prostate mpMRI via Combining Intensity and Shape Information1GE Global Research Center, Niskayuna, NY, United States, 2GE Global Research Center, Bengaluru, India, 3Memorial Sloan-Kettering Cancer Center, New York, NY, United States
Synopsis
In prostate MRI, tissue alignment between T2w and DWI can be challenging due to elastic distortions in DWI induced by fast switching of gradients. In this work, we propose a joint approach to perform registration between DWI and T2w on the prostate data, by considering both image intensity and prostate gland shape information. After rigid alignment between segmented prostate masks, surface points on the prostate masks were extracted and Gaussian mixture model was used to build shape correspondence. The shape information was integrated into mutual-information based deformable registration to constrain undesired distortions. The proposed framework was compared with other strategies and demonstrated the best registration accuracy (DICE = 0.91 between T2 and DWI based prostate masks).
PURPOSE
Multi-parametric magnetic resonance imaging (mpMRI) has shown increased sensitivities in prostate cancer (PCa) detection [1]. Moreover, PI-RADSv2 guidelines have emphasized on using the T2-weighted (T2w) and diffusion weighted imaging (DWI) data for scoring PCa [2]. Accurate mpMRI registration allows for spatial comparison and lesion detection at the voxel level. However, multimodal prostate registration is challenging due to various factors, e.g., large differences in scan resolution, FOV, imaging planes, patient motions, etc. Specifically, tissue alignment between T2w and DWI can be challenging due to elastic distortions in DWI induced by fast switching of gradients [3]. In this study, we presented a framework to perform non-rigid registration between DWI and T2w on the prostate MRI, by considering a joint image intensity and prostate gland shape information during registration.METHODS
30 PCa patients with Gleason score 6 to 9 were enrolled in an IRB-approved study and both the T2w and DWI scans were acquired on a 3T DISCOVERY MR 750 MRI scanner (GEHC, Waukesha, WI, USA) with endorectal coil. The MRI protocol included a. T2w: axial oblique, TE/TR = 116/5614 ms, resolution = 0.3125 × 0.3125 × 3mm3, matrix = 512 × 512 × 30~45 and b. DWI: axial oblique, TE/TR = 104/3500 ms, resolution = 0.65 × 0.65 × 3 mm3, matrix = 256 × 256 × 30~45 and b-values of 0 and 1000 s/mm2.
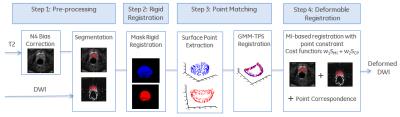
Figure 1 shows the flowchart of the proposed framework. First, the T2w data was corrected for intensity bias using N4 algorithm [4]. Next, the boundary of the prostate gland was manually delineated on both T2w and DWI scans. A rigid body registration was employed on the segmented prostate masks to register DWI to T2w through minimizing the sum of squared differences and the rigid transformation was applied to the DWI images. Surface points from both T2w and rigidly aligned DWI masks were extracted and those two set of points characterized the prostate shape and topology. Their correspondence was created via a point matching algorithm [5], which represented point sets using Gaussian Mixture Model (GMM) and considered the registration problem as the problem of aligning two Gaussian mixtures. Finally, the point correspondence was integrated into the mutual information (MI) based registration and both image intensity and prostate gland shape drove the nonrigid registration [6]. Hence, the registration not only maximized the MI metric, but also considered the corresponding positions. In addition, spatial samples used to calculate the MI were selected from the T2w prostate mask, instead of from the whole image.
The proposed framework was compared with other two strategies: the nonrigid registration neither selecting spatial samples from the mask nor using the point correspondence, and the nonrigid registration with the samples from the mask but without the point constraint. The DICE overlap metric between T2w and DWI prostate gland masks was calculated to evaluate the registration. To provide a fair comparison, same registration parameter setting was used for different strategies and for all subjects.
RESULTS
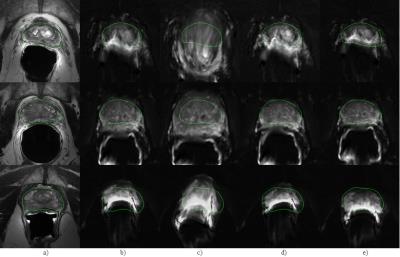
Figure 2 shows registration results for three representative cases. Columns a – e display T2w with the prostate gland mask, DWI after rigid registration, DWI after registration w/o using masks and points, DWI after registration w/o points, and DWI after the proposed joint approach, respectively. The contours in the T2w images were copied to other panels to facilitate the comparison. Without the mask and point constraints, the DWI images were distorted substantially due to the poor contrast in DWI and bad anatomical structure correspondence between DWI and T2w. With the mask, the alignment was better but still not accurate. The proposed method yielded the best registration result.
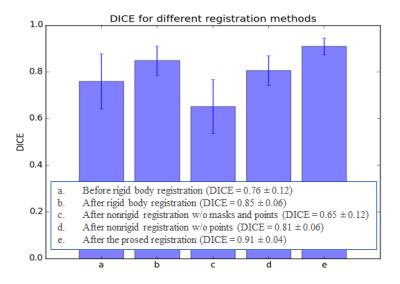
Figure 3 plots the mean DICE values of all 30 subjects for various registration schemes. The mean DICE indexes were 0.76 before registration, 0.85 after rigid registration, 0.65 after registration without any constraints, 0.81 without the point constraints, and 0.91 after the proposed method, respectively. The standard deviations also demonstrated that the proposed method outperformed other methods.
DISCUSSION
The study presented a DWI-T2w registration approach in which the prostate gland points provided additional shape information during the intensity-based registration and improved the registration accuracy. The framework is fully automated except for the prostate segmentation. Fully automatic prostate segmentation is still a challenging problem and the state-of-the-art methods include the active appearance model, atlas-based segmentation, and discriminative learning [7]. Future work includes conducting a more comprehensive validation (e.g., investigating the Dice values for the peripheral zone and transition zone), and extending the approach through utilizing a multivariate format of both MI and shape information for multimodal data.Acknowledgements
No acknowledgement found.References
1. Hoeks CMA et al., Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology 2011;261(1):46–66.
2. https://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/PIRADS/PIRADS%20V2.pdf.
3. Le Bihan D, Artifacts and pitfalls in diffusion MRI. J Magn Reson Imaging 2006;24(3):478 –488.
4. Tustison NJ, Avants BB,Cook PA, et al., N4itk: improved n3 bias correction. IEEE Trans Med Imaging 2010;29:1310–1320.
5. Jian B, Vemuri BC, Robust Point Set Registration Using Gaussian Mixture Models. IEEE PAMI 2011;33:1633–1645.
6. Klein S, Staring M, Murphy K, et al., elastix: a toolbox for intensity-based medical image registration. IEEE Trans. Med. Imaging 2010;29:196–205.
7. Litjens G, Toth R, van de Ven W et al., Evaluation of prostate segmentation algorithms for MRI: the PROMISE12 challenge, Medical Image Analysis 2014;18:359–373.
Figures