3926
Robust EEG-fMRI using optical motion tracking: Retrospective EEG Motion Educated Gradient Artefact Suppression REEG-MEGAS1Department of Medicine, John A. Burns School of Medicine, University of Hawaii, Honolulu, HI, United States, 2Developmental Imaging and Biophysics Section, University College London, London, United Kingdom
Synopsis
Here we present a method capable of suppressing motion-related EEG GA instabilities, induced EEG voltages and fMRI artefacts in simultaneous EEG-fMRI data. This correction method appeared to allow for the removal of harmonics associated with incomplete GA removal even in a moving subject. Therefore our findings might be helpful in improving both EEG quantification reliability and data quality in populations prone to movement e.g. children or for studying patients with epilepsy during seizures.
Purpose
Simultaneous EEG-fMRI is an important tool to study brain function in health and disease across spatial and temporal scales. Prospective Motion Correction (PMC)1 by tracking subject position can dramatically increase fMRI data quality and may have an important application in populations where movement is hard to prevent such as studies in children. We have recently demonstrated that a PMC system, while suppressing fMRI motion artefacts, can be used for Retrospective EEG Motion Artefact Suppression (REEGMAS)2. This was achieved by regression of the EEG using a model (six translations/rotations, their velocities and velocities2) of motion-induced voltages within the main magnetic field. In this work we extend the model to additionally correct for non-stationarity in magnetic field gradient induced EEG artefacts (GA) by combining REEGMAS with a template subtraction3 within the framework of a general linear model.Methods
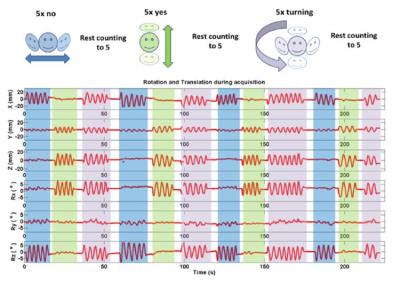
Simultaneous EEG-fMRI data were acquired in a 1.5T Avanto scanner (Siemens, Germany) using a MRI compatible amplifier (Brain Products, Germany) with a 31-channels+ECG cap (Easy-cap, Germany). The EEG data were acquired with a sampling rate of 5kHz and were downsampled to 500Hz. We acquired 30 slices (slice TR=74ms), 100 volumes (222s) of functional images using a gradient-echo echo-planar imaging (GE-EPI) sequence, for more details see2. An MR-compatible camera (Metria, USA) was used to track a Moiré Phase Tracking (MPT) marker1 attached to a ‘bite bar’ specifically developed for each subject based on a dental retainer. Motion was tracked through-out but fMRI PMC was turned on and off. We acquired 4 fMRI runs: subjects keeping still: 1-PMC-on and 2-PMC-off; Subjects moving their head (Fig. 1): 3-PMC-on and 4-PMCoff. We also acquired EEG data from outside the scanner with the subject keeping still (Baseline). For the keeping still sessions the subjects were instructed to switch between eyes opened/closed every 40s, and for the moving sessions they switched every time they finished a motion cycle (‘no’, ‘yes’ and ‘turning’, Fig. 1).
Retrospective EEG Motion Educated Gradient Artefact Suppression (REEG-MEGAS):
Template artefact suppression (AAS) can be viewed as a GLM where each column is a stick function with ones at the temporal positions where the GA is thought to repeat (here the slice TR). To account for changes in the GA due to the variable subject head position and velocity this is convolved with the REEGMAS model (translation/rotations, their derivatives and derivatives2) thereby modelling both the motion induced voltages (as for REEGMAS) but additional motion related variability in the GA. To reduce the potential effect of motion measurement error the GLM is estimated using a robust least squares approach as implemented in ‘robustfit.m’. This new approach (REEG-MEGAS) was compared to a standard AAS both with and without REEGMAS motion voltage correction.
Results
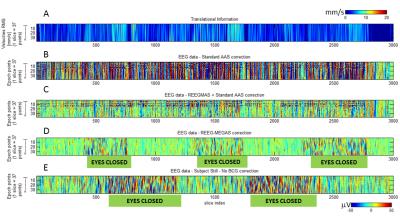
The EEG data (Fig. 2 B) with standard AAS correction is strongly affect by motion-induced artefacts which are reduced by applying REEGMAS (Fig. 2C). However GA residuals are clearly visible as horizontal structure indicating it is at the same point in the TR (lines 8 to 15, Fig. 2B and C). REEG-MEGAS provides data less affected by GA residuals and motion induced artefacts (Fig. 2 D), resulting in data comparable to that acquired in the keeping still runs (Fig. 2E). Besides that the epochs when the subjects were with their eyes closed (with alpha rhythm presence) are easier to identified, suggesting the improvement off the signal to noise ratio (SNR) of the data.
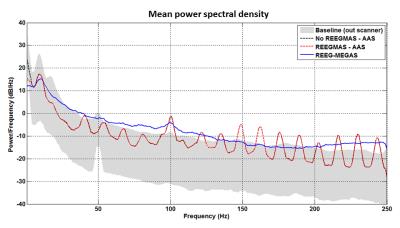
The PSD analysis considering the eyes closed epochs supports that the SNR is improved for the alpha rhythm by using Robust AAS (Fig. 4) when it is compared to the other approaches. Additionally, the Robust AAS also suppress the GA harmonics usually presented in higher frequencies (>40Hz). We verified similar results for both subjects in both conditions (with and without PMC on fMRI).
Discussion
EEG-fMRI data can be strongly affected by motion due to voltage induced artefacts but it also increases GA instability. Our results show that both effects can be suppressed by applying REEG-MEGAS (Fig. 2D). The Robust AAS also suppresses the higher frequency GA harmonics (Fig. 3) which may be of interest for studying electrophysiological features at high frequencies.Conclusion
A method capable of suppressing motion-related EEG GA instabilities, induced EEG voltages and fMRI artefacts in simultaneous EEG-fMRI data was proposed. This correction method appeared to allow for the removal of harmonics associated with incomplete GA removal even in a moving subject. Therefore our findings might be helpful in improving both EEG quantification reliability and data quality in populations prone to movement e.g. children or for studying patients with epilepsy during seizures.Acknowledgements
No acknowledgement found.References
1 - Maclaren J, Herbst M, Speck O, Zaitsev M. Prospective motion correction in brain imaging: a review. Magn Reson Med. 2013;69,621–636.
2 - Maziero D, Velasco TR, Hunt N, et al. Towards motion insensitive EEG-fMRI: Correcting motion-induced voltages and gradient artefact instability in EEG using an fMRI prospective motion correction (PMC) system. NeuroImage. 2016;138,13–27.
3 - Allen PJ, Josephs O, Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. NeuroImage. 2000;12,230–239.
Figures