3923
A within-subject comparison of anatomical and diffusion scans from Siemens TimTrio and Prisma scanners1Center for Brain Science, Harvard University, Cambridge, MA, United States, 2Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States
Synopsis
Siemens' latest 3 Tesla scanner, the Magnetom Prisma, represents a significant upgrade in performance capability over the Magnetom TimTrio. To quantify the improvements offered by such a significant system upgrade, we scanned 8 subjects using a variety of anatomical, functional and diffusion protocols on the TimTrio platform, and then repeated the same protocols on the Prisma platform with the same subjects after the upgrade process. We found consistency in morphometric results from anatomical scans acquired using recommended T1-weighted imaging protocols. Modest improvements in tSNR for high-resolution and highly-slice accelerated BOLD scans were seen, but more traditional 3mm resolution scans yielded no improvement presumably due to the dominance of physiological noise. The DTI scans conducted here benefit greatly from the new gradient coil in the Prisma, when protocols are optimized to reduce TE and bandwidth as allowed by the new gradient set.
Introduction
Siemens' latest 3 Tesla scanner, the Magnetom Prisma, represents a significant upgrade in performance capability over the Magnetom TimTrio. The Prisma includes a major advance in gradient strength (80 mT/m), new highly-parallel array receive coils for the head, a digital RF transmit/receive architecture, and a much faster reconstruction computer. The new gradient coil is expected to offer significant improvements in diffusion experiments, while the digital RF has the potential to improve RF fidelity and reduce spurious noise pick-up, resulting in improved image SNR. However, for longitudinal studies, consistency in basic anatomical scanning is important. As the magnet dimensions are the same, the TimTrio can be directly upgraded to the Prisma platform without replacing the magnet. To quantify the improvements offered by such a significant system upgrade, we scanned 8 subjects using a variety of anatomical, functional and diffusion protocols on the TimTrio platform, and then repeated the same protocols on the Prisma platform with the same subjects after the upgrade process.Methods
8 subjects were scanned on the Siemens 3T Tim Trio scanner before it was upgraded to the Prisma platform. The same 8 subjects were scanned a second time on the Siemens 3T Prisma, after the scanner conversion. The relevant 32-channel head coil was used on each system. The scans included 1.0mm and 1.2mm resolution multi-echo MPRAGE1 anatomical scans; 8-min duration resting state BOLD scans, one with 3mm resolution and TR=3s, one with 2mm nominal resolution, slice-acceleration2,3 (SMS) of 8 and TR=750ms; and two DTI protocols - both with nominal 2mm resolution: no SMS, b=1000s/mm2, 30 directions; and SMS 2, b=1000s/mm2, 64 directions. On the Prisma, the DTI protocols were reproduced exactly as implemented on the Trio, and then repeated optimizing the echo spacing and minimizing TE and TR as allowed by the Prisma gradient set. Anatomical scans were analyzed using Freesurfer4 v.5.3, BOLD scans analyzed by assessing tSNR for each voxel, and correlations between major network seeds5. DTI scans were analyzed from ADC and FA maps generated by the scanner software, and offline after detailed eddy-current and distortion correction using FSL tools, registration to T1-weighted image space, alignment with white-matter structures, and calculation of signal stability from b=0 images.Results
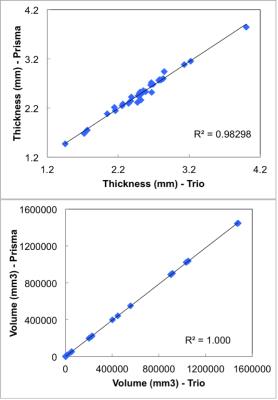
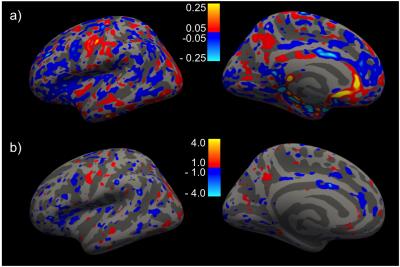
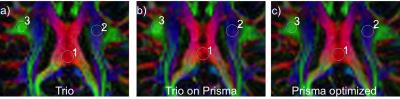
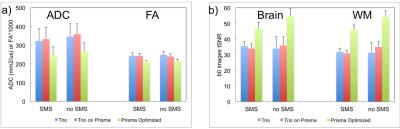
Fig. 1 shows correlation between the two scanners for the (a) thickness of all 33 cortical regions of the Desikan-Killiany atlas, and (b) volume of all sub-cortical structures, from a single subject. Within subject for all regions, or across subjects for a given region/measure, correlation is generally very high. Fig. 2 shows inflated-surface plots of (a) cortical thickness difference; and (b) significance of thickness differences; for groups of scans on the Trio and the Prisma. Thickness differences are thresholded at ± 50μm; significance plots to p < 0.1. The group average, whole-brain tSNR for each of the resting-state functional scans did not increase for the 3mm/no-SMS protocol, while a 20% increase in tSNR was seen for the 2mm/SMS8 protocol. The tSNR increase did not generally yield in improvement in network definition, however. FA maps from the DTI scans showed clear improvement simply from visual observation at the scanner console. Fig. 3 shows a portion of the color-coded FA map from the SMS2 DTI scan on the Trio and the two corresponding scans on the Prisma. Standard-deviation of ADC and FA in the marked ROI's is noticeably lower for the Prisma-optimized DTI protocol, while diffusion signal stability is in increased (Fig. 4).Discussion
This within-subject study before and after the Prisma upgrade shows consistency in morphometric results from anatomical scans acquired using recommended T1-weighted imaging protocols, after correction for gradient non-linearity on the two systems. We saw modest improvements in tSNR for high-resolution and highly-slice accelerated BOLD scans, but more traditional 3mm resolution scans yielded no improvement presumably due to the dominance of physiological noise. The DTI scans conducted here benefit greatly from the new gradient coil in the Prisma, when protocols are optimized to reduce TE and bandwidth as allowed by the new gradient set. Otherwise, results are consistent with those from Trio, when protocols are implemented identically. However the major benefit should be seen in higher b-value and multi-b shell scans that are rarely contemplated in a routine environment.Acknowledgements
Harvard Center for Brain Science; NIH Shared Instrumentation Grant S10OD020039; NIH Grants P41-RR14075, U24-RR021382References
1. van der Kouwe, A.J.W. et al. (2008), Brain morphometry with multiecho MPRAGE, NeuroImage, vol. 40, pp. 559–569.
2. Moeller S, et al (2010), Multiband multislice GE-EPI at 7 Tesla with 16-fold acceleration using Partial Parallel Imaging with application to high spatial and temporal whole-brain fMRI, Magn. Reson. Med., vol. 63, pp. 1144–1153.
3. Setsompop K, et al (2012), Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty, Magn. Reson. Med., vol. 67, pp. 1210-1224.
4. Fischl, B. (2000), Measuring the thickness of the human cerebral cortex from magnetic resonance images, Proc. Natl. Acad. Sci. USA, vol. 97, pp. 11050-11055.
5. Yeo, B.T.T. et al. (2011), The organization of the human cerebral cortex estimated by intrinsic functional connectivity, J. Neuropyhsiol., vol. 106, pp. 1125-65.
Figures