3912
Quality Assurance Phantoms and Procedures for UHF MRI ‒ The German Ultrahigh Field Imaging (GUFI) Approach1Erwin L. Hahn Institute for Magnetic Resonance Imaging, University of Duisburg-Essen, Essen, Germany, 2German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 3Otto-von-Guericke-University, Magdeburg, Germany, 4Medical Physics in Radiology, German Cancer Research Center (dkfz), Heidelberg, Germany, 5High Field and Hybrid MR Imaging, University Hospital Essen, University of Duisburg-Essen, 6Leibniz Institute for Neurobiology, Magddeburg, Germany
Synopsis
The German Ultrahigh Field Imaging network (GUFI, www.mr-gufi.de) is a user group of 13 German and neighboring sites that all operate a UHF (7T or 9.4T) MRI system. Due to the lack of common quality assurance (QA) procedures for UHF, GUFI started an initiative to unify QA procedures at these sites. A QA phantom and measurement protocol were developed especially for UHF that is currently being rolled out to all member sites. The QA data allow monitoring of individual system performance based on long-term data analysis or by comparison to pooled data from all sites.
Purpose
In this study we developed a quality assurance (QA) phantom and measurement procedure for UHF MRI. We collected QA data from four sites equipped with 7T systems and compared their key system-related performance parameters. The proposed method should allow assessment of system performance and comparison to other UHF MRI scanners.Material and Methods
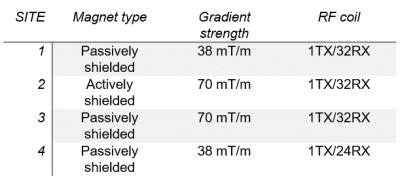
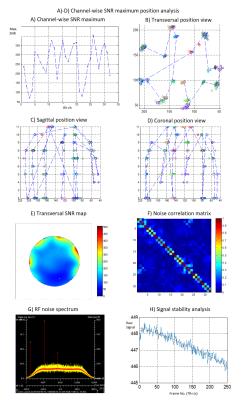
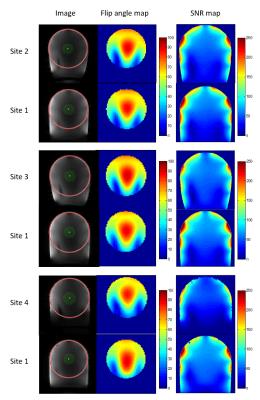
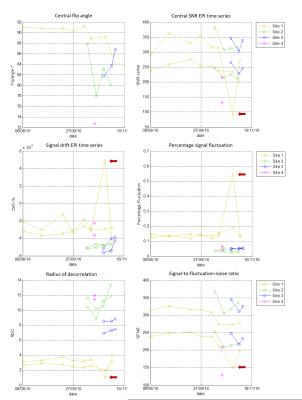
The QA phantom needs a stable filling material with dielectric properties similar to human tissue. Therefore, a dilution series for polyvinylpyrrolidone (PVP) and salt in water was mixed to find a recipe for the modification of the dielectric properties suitable for both 7T and 9.4T MR systems. This recipe was used to calculate the concentrations needed for the phantom fluids. The housing of the phantom was made with two compartments. The head-and-neck part is formed from a PMMA tube terminated on the two ends with a half shell and a plate, respectively (Fig 1). This container was fitted into a widely available 1TX/32RX-channel RF head coil (Nova Medical, MA) and filled with a gel of water, PVP, agar, and salt to emulate brain tissue. The second part of the phantom was designed to emulate the load of the human shoulders on the RF coil. Therefore, a rectangular container was fitted adjacent to the coil and head-and-neck part (Fig 1). This container was filled with a solution of PVP and salt to emulate muscle tissue. Four of the QA phantoms were fabricated, and QA analysis has started at four different UHF sites. All sites are equipped with a whole-body 7T MRI (Siemens Healthcare), but some major hardware components, i.e. the magnet, the gradient coil, and the RF coil differ between the sites. (Fig 2 / Table 1). A measurement protocol was created to check for RF coil performance as well as gradient and system stability; in addition, the protocol was designed to verify the stability of the phantom filling material. RF coil performance was measured with B1, SNR, and coupling measurements like noise correlation or SNR maximum position analysis (Fig 3). Using noise measurements, the RF system was checked for unwanted noise sources and the gradients / system were checked for RF spikes. The system was stressed with high-duty-cycle EPI bold imaging to capture stability parameters, e.g. signal drift or fluctuation according to the recommendations of the fBIRN consortium1. Relaxometry with multi-echo spin-echo and inversion recovery spin-echo measurements were appended to confirm the long-term stability of the phantom. From the acquired images, parameters such as central SNR or flip angle, noise correlation, system drift, and fluctuation or relaxivity were calculated; the data were analyzed across longitudinal measurements and between sites. Before delivery, all phantoms underwent a complete QA measurement with this QA protocol at the same site (Fig 4).
Results
Based on dielectric and relaxation properties, the phantom fluid was suitable to emulate the desired tissues. The phantoms had similar relaxation behavior at all sites (mean: T1 = 680ms, all deviations <= 10%, T2 = 58ms, dev. <= 3%). The mean relative permittivity of all phantoms was 55.5 (dev. <= 4%), and the mean conductivity was 0.67 S/m (dev. <= 8%). Analysis across sites revealed high agreement regarding basic image parameters such as SNR and achievable flip angles if an RF coil of the same type was used. Differences caused by the different models of the gradient and RF coils can easily be identified (Fig 4). The inter-site differences are in the same range as the differences found in longitudinal data analysis (Fig 5). System stability parameters revealed differences between the sites, and longitudinal data confirmed these findings (Fig 5).Discussion and Conclusion
Acknowledgements
The research leading to these results has received funding from the German Research Foundation (DFG) / project German Ultrahigh Field Imaging / Grant n. LA 1325/5-1References
1. Friedman et al., JMRI 23:827–839 (2006)Figures