3911
Free breathing Motion-Robust Cardiac B1+ mapping at 3.0T based on DREAM1Technische Universität München, Munich, Germany, 2GE Global Research, Munich, Germany, 3Cardiac Center of Excellence, GE Healthcare, Munich, Germany, 4GE Healthcare, Waukesha, WI, United States
Synopsis
Radiofrequency (RF) field inhomogeneities affect image quality while performing body and cardiac imaging especially at high field strengths. RF shimming and quantitative MR applications such as T1 mapping can potentially benefit from an accurate knowledge of variations of the transmitted RF field (B1+).
The purpose of this work is to investigate spatial homogeneity of the B1+ in the myocardium at 3.0T. For this study, we developed a prospective ECG-gated method based on DREAM (Dual Refocusing Echo Acquisition Mode).
The presented approach allows free breathing multi-shot acquisition B1+ mapping and its feasibility is shown in vivo.
PURPOSE
Clinically relevant applications of cardiac MRI like quantitative tissue characterization of the myocardium require an accurate knowledge of the uniformity of the transmitted RF (B1+) field, especially at high field strengths. Several studies have reported that B1+ inhomogeneities can lead to image artifacts and thereby compromise diagnosis 1-3.
However, quantification of cardiac B1+ maps remains challenging due to cardiac and respiratory motion and, moreover the need of time-efficient and motion-robust sequences.
In the present work, we propose a fast multi-single shot B1+ mapping method, based on the DREAM sequence 4 and prospectively synchronized with the cardiac cycle to measure and characterize static and dynamic field inhomogeneities within the myocardium.
As reported in other cardiac B1+ studies 5 the specific absorption rate (SAR) could be a limiting factor when using the Bloch-Siegert Shift. The DREAM method derives B1+ maps from SPGR images acquired with a low flip angle train, therefore the SAR burden is low making it a promising B1+ mapping solution for ultrahigh-field systems.
MATERIALS AND METHODS
In-vivo experiments were performed on a 3.0T system GE MR750w (GE Healthcare, Milwaukee, WI) using the standard birdcage body coil in quadrature mode for RF transmission and 20-channel torso array coil for signal reception. B0 shimming was applied using the default techniques supplied by the vendor.
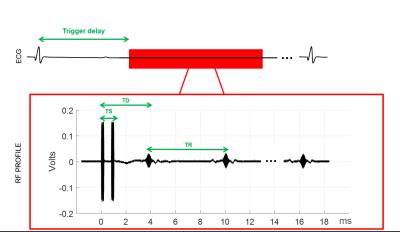
B1+ maps were obtained using the modified DREAM sequence for a short axis orientation according to the cardiac imaging standards. For each slice, prospective ECG-gating allowed imaging during the diastolic quiescence of a single heartbeat. The average heart rate for the subject was 50 bpm.
The virtual stimulated DREAM echo scheme (STE*) was used. Figure 1 shows the basic pulse sequence scheme and acquisition window. Scans were acquired with the following imaging parameters: TR/TS/TD =6.2ms/0.8ms/3.9ms; STEAM flip angle α= 60°, imaging flip angle β= 15°, matrix size= 96x96; slice thickness=10mm; Phase FOV=0.8; BW=15kHz; Partial Fourier (NEX=0.5) was performed to minimize total acquisition time (380 ms) and centric order was chosen to reduce intra-acquisition motion artefacts.
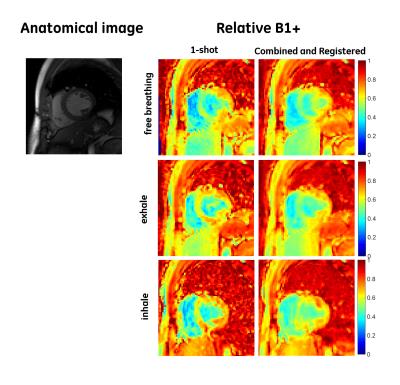
Ten repeated single-shot B1+ maps were acquired to study reproducibility in free breathing and breath-held positions (inhale and exhale). Native STE and FID echoes which are used to derive B1+maps 4, were registered and combined to obtain motion insensitive B1+ maps with higher SNR. All computations were performed using MATLAB (The Mathworks, Natick, MA, USA).
RESULTS
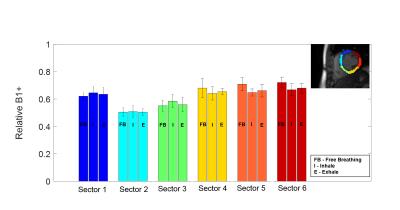
In vivo relative B1+ maps, calculated as a fraction of intended flip angle are shown in Figure 2 for different respiratory positions and reconstruction methods. Mean values and standard deviations in myocardium were calculated within six ROI’s manually drawn as defined by AHA 6. High consistency of B1+ values is observed between free-breathing and breath hold (inhale/exhale) states which indicates motion robustness of this approach (see Figure 3).
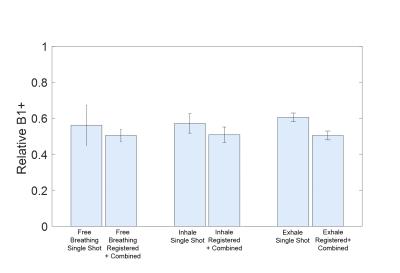
As depicted in Figure 4 the standard deviation of B1+ values within a representative ROI is demonstrating the impact of registration and combination of multiple shots compared to single shot B1+ maps.
Variations between basal anteroseptal and anterolateral segments (segment2/segment 6) of 34% (free breathing), 25 % (inhale), 28% (exhale) were observed (Figure 3) showing strong agreement with previous studies 7.
DISCUSSION AND CONCLUSION
Here we demonstrated in vivo feasibility of a cardiac gated multi single shot B1+ mapping, which allows motion artifact-free mapping.
Cardiac motion is addressed by synchronizing acquisition with the cardiac cycle using a physiological trigger device (prospective ECG gating).
Furthermore, multiple single shot acquisitions are expected to improve robustness to respiratory motion which is a clear advantage compared to previously published studies where acquisition of two separate images and breath-holding are required 7,8.
Moreover, the proposed method performs a SPGR Cartesian k-space readout, as commonly used in clinical CMR sequences. This confers robustness against acquisition specific artifacts, e.g. radial/spiral while relying on standard reconstruction techniques available in most MRI systems and could be easily integrated into the clinical workflow.
The proposed method is particularly interesting for cardiac B1+ mapping at high fields due to its low SAR burden.
Although studying B0 changes was beyond the scope of this study the DREAM approach provides simultaneous B0 maps and could be beneficial for other applications.
Due to flow effects, reliable values in the blood pool of the left ventricle are still challenging. However, flow compensation, a smart undersampling strategy across shots and further validation in a larger group may help and will be investigated in future research.
Acknowledgements
This publication was supported by the European Commission, through Grant Number 605162. The content is solely the responsibility of the authors and does not necessarily represent the official views of the EU.
The authors gratefully thank the assistance and fruitful discussions with Prof. Burschka, Prof. J. Felblinger, Dr. F. Odille and Dr. P.-A. Vuissoz.
References
1. Mueller et al. Dual Source Radiofrequency Transmission with Patient-Adaptive Local Radiofrequency Shimming for 3.0-T Cardiac Imaging: Initial Experience. Radiology: volume 263: Number 1 (2012): 77-85.
2. Cloos, M. A.; Knoll, F.; Zhao, T. Block, K. T.; Bruno, M.; Wiggins, G. C. & Sodickson, D.K. Multiparametric imaging with heterogeneous radiofrequency fields. Nat Commun, 2016, 7, 12445.
3. Kim D, Cernicanu A, Axel L. B0 and B1-insnsitive uniform T1 weighting for quantitative, first pass myocardial perfusion magnetic resonance imaging. Magnetic resonance in Medicine (2005); 56:1423-1429.
4. Nehrke, K. and Börnert, P. DREAM—a novel approach for robust, ultrafast, multislice B1 mapping. Magnetic Resonance in Medicine, 68 (2012); 1517–1526.
5. Weingaertner S. et al. Motion-Robust Cardiac B1+ mapping at 3T using Interleaved Bloch-Siegert Shifts. Magnetic resonance in Medicine (2016); 00-00.
6. M. D. Cerqueira. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American Heart Association. Circulation, 105(4):539–542, January 2002.
7. Kyunghyung S. and Nayak K.S. Measurement and Characterization of RF Uniformity over the Heart at 3T. Journal of Magnetic Resonance Imaging, 27. (2008); 643-648.
8. Clique H, Cheng HL, Marie PY, Feblinger J, Beaumont M. 3D Myocardial T1mapping at 3T using variable flip angle method: pilot study. Magnetic resonance in Medicine (2014); 71 (2): 823-9.
Figures