3901
Automatic Extraction of Cardiac and Respiratory Motion via Self-Refocused Rosette Navigators and Independent Component Analysis1Radiology, Center for Advanced Imaging Innovation and Research (CAI2R), New York University Langone Medical Center, New York, NY, United States, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Siemens Healthcare, New York, NY, 4European Institute for Molecular Imaging, University of Mϋnster, Mϋnster, Germany
Synopsis
Due to the recent availability of simultaneous PET-MR, there has been much interest in MR-based motion correction for PET imaging. A key component of any such scheme is a mechanism for tracking respiratory and cardiac motion phases throughout the entire exam. In this work, we present a robust, automated approach whereby respiratory and cardiac motion information is jointly encoded with rosette navigators and decoded via independent component analysis (ICA). This approach obviates the need for any external motion tracking devices (e.g. bellows or ECG) and requires just a contrast-neutral, self-refocused navigator echo (≈2ms) per repetition time and so may be easily incorporated into many clinical sequences.
Introduction
Simultaneous PET-MR scanners provide an ideal platform for removing respiratory and cardiac motion from simultaneously acquired PET images. One appealing approach is to develop an MR-based motion model during the first few minutes of the scan and then to apply this motion information to correct the entirety of the PET listmode data [2]. However, to apply the motion model to the PET data, one requires some means to track the respiratory and cardiac phases throughout the entire examination, so that the PET listmode data can be grouped into motion states. MR-based, self-refocused navigators have been proposed to achieve this goal [3] because they can effectively encode motion information without affecting the underlying image contrast and can, therefore, be incorporated into a large variety of sequences.In this work, we present a robust approach for obtaining both respiratory and cardiac gating information from self-refocused, rosette navigators using independent component analysis (ICA) [5]. This framework automatically separates the desired signals from noise and artifacts without any need for model training or a priori information. Our approach is demonstrated on four human subjects as well as on an MRI-compatible motion phantom (Wilhelm MRI phantom [7]) that simulates cardiac and respiratory motion and provides a ground truth measurement of diaphragm displacement.Methods
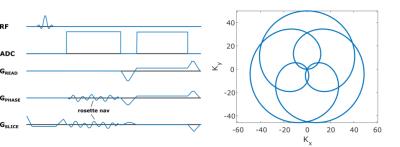
All scans were performed on a Siemens Biograph mMR (Siemens Healthcare, Erlangen, Germany) using the body coil array and a golden angle radial imaging (RI) in-house sequence (TR/TE=12.7/5ms, BW=488Hz/Pixel, 80x4.5mm slices, 800 radial views, Figure 1), modified to incorporate a self-refocused, rosette [4] navigator readout.The k-space data were then sorted into a 2D matrix; where each column is formed by concatenating the rosette-navigator readout from all coils, and the total number of columns is equal to the number of repetitions. This matrix is processed in MATLAB using the fastICA algorithm [6]. The first step of the algorithm involves prewhitening the data via principal component analysis (PCA), whereby the dimensionality is reduced (typically to between 8-20) by excluding components with eigenvalues λ/λmax < 1%. Subsequently the independent components (IC’s) are discovered by seeking an ND rotation of the prewhitened data that maximizes the “non-Gaussianity” of the components. IC’s corresponding to respiratory and cardiac motion are identified by taking the IC with the largest fraction of its power in a prespecified frequency band (0.1-0.5Hz for respiratory and 0.75-1.5 Hz for cardiac).Results
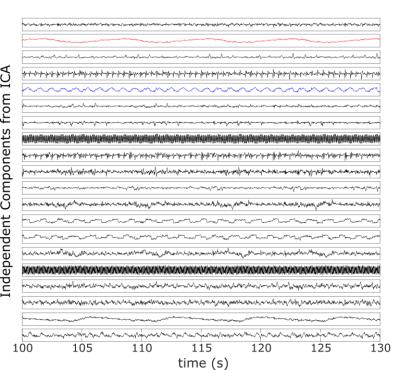
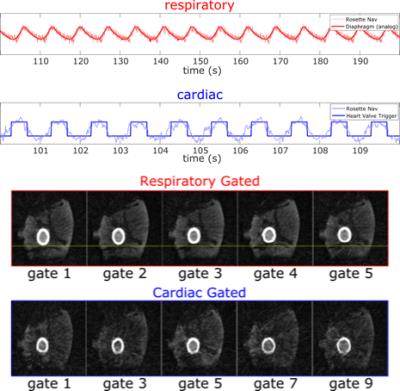
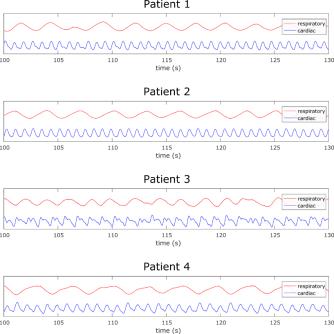
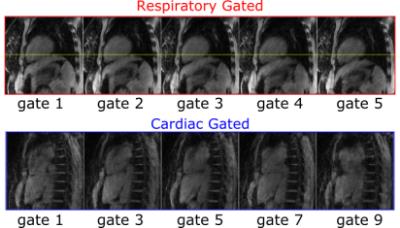
Figure 2 depicts the 20 IC’s obtained by processing the rosette-navigator readouts with the fastICA algorithm for the Wilhelm phantom scan. Among them are various artifacts from gradient switching as well as the desired respiratory (red) and cardiac (blue) gating signals, which have been identified automatically as discussed above. Figure 3 compares estimated (blue) and ground truth (red) respiratory displacements for the diaphragm of the Wilhelm phantom. Also, the cardiac gating signal matches up well with the triggering signal for the heart valve. Furthermore, the programmed heart rate of the Wilhelm phantom is 60 bpm (1 Hz), which matches exactly the peak frequency of the cardiac gating signal obtained from the navigator. Figure 4 depicts similar respiratory (red) and cardiac (blue) gating signals obtained from 4 human subjects. Figure 5 shows corresponding MR reconstructions for cardiac and respiratory states obtained for one of the Patients.Conclusion
We have demonstrated a robust, automated approach to obtaining respiratory and cardiac gating signals via self-refocused, rosette navigators combined with ICA. We also validated our approach with the dynamic Wilhelm phantom, showing that the obtained gating signals match extremely well with the ground truth. The only prior information required is a loose bound on the frequency of the desired gating signal. The navigator used in this study could potentially be incorporated into a wide variety of imaging sequences, allowing for continuous motion tracking throughout an MR examination. Furthermore, the proposed navigator requires only an additional 2ms per repetition and does not affect the underlying image contrast, making it a flexible, attractive tool for PET-MR motion correction.Acknowledgements
We would like to thank Li Feng and Ricardo Otazo (Center for Advanced Imaging Innovation and Research , New York University Langone Medical Center, New York, NY, USA) for sharing MRI reconstruction code.References
[1] Peters D. C., et al., Magn Reson Med. 2000, 43: 91-101.
[2] Koesters T., et al., Proc. ISMRM 2014, pp. 789.
[3] Vahedipour K., et al., Proc. ISMRM 2015, pp. 813.
[4] Noll D. C., et al., Magn Reson Med. 1998, 39: 709-16.
[5] Jutten, C. et al., Signal processing. 1991, 24.1: 1–10.
[6] Hyvarinen, A., IEEE transactions on neural networks. 1999, 626–634.
[7] Fieseler M et al., Nucl. Inst. and Meth. in Physics and Research A, 2015, (702): 59-63
Figures