3900
MR-Based Respiratory and Cardiac Motion Corrected 18F-FDG-PET/MR in Cardiac Sarcoidosis1Translational and Molecular Imaging Institute, icahn school of medicine at mount sinai, New York, NY, United States, 2Department of Nuclear Medicine, European University of Brittany, 3British Heart Foundation/University Centre for Cardiovascular Science, University of Edinburgh, 4Cardiovascular Institute, Icahn School of Medicine at Mount Sinai
Synopsis
A major advantage of hybrid PET/MR systems is the radiation-free high spatial and temporal resolution of cardiac MR imaging that can be used to estimate respiratory and cardiac motion present during PET data acquisition. This information can be incorporated into algorithms to correct for the effects of motion in the PET data to reduce blurring and increase target-to-background ratios of PET hotspots. In this work, we demonstrate a method for respiratory and cardiac motion correction in patients with cardiac sarcoidosis.
Introduction
Hybrid PET/MR scanners offer the opportunity to obtain spatially and temporally co-registered images with the advantages of both modalities. Hybrid PET/MR has significant potential in cardiac applications [1] where both functional changes and the pattern of injury can be determined by cine- and late gadolinium enhancement (LGE)-MRI and the activity of disease can be measured by PET. Sarcoidosis is a granulomatous disease that can affect the heart. Prognosis is poor once sarcoidosis involves the heart, however, treatment can be effective, therefore optimal diagnostic imaging is important. Currently, active cardiac sarcoidosis is assessed by identifying inflammation in the myocardium using 18F-fluorodeoxygluocose (18F-FDG)-PET/CT. Substantial respiratory and cardiac motion during PET data acquisition can cause both image blurring and diminution of target-to-background ratios, potentially reducing sensitivity for detection of disease. In this work we utilize 3D temporally resolved MRI to estimate respiratory and cardiac motion and apply this to correct for motion in double-gated PET data.Methods
A patient with proven extra-cardiac sarcoidosis and suspected cardiac involvement underwent hybrid PET/MR (Biograph mMR, Siemens) to test the feasibility of MR-based motion correction of the PET data. 18F-FDG (10 mCi) was administered. After patient set-up and initial image scouts, list-mode PET data acquisition began after 30 minutes and lasted for 60 minutes.
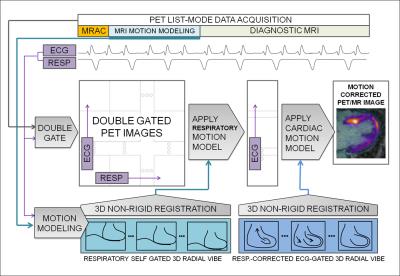
Motion Estimation: Respiratory motion was estimated using a free-breathing 3D golden-angle radial VIBE sequence (based on Siemens WIP-793, [2]). A whole-body coronal slab with 3x3x3mm-resolution and 1600 spokes was acquired over 6-7min. The amplitude of the center of k-space was extracted from the raw data and plotted over time. This quantity was taken to reflect the volume of liver in the field of view and subsequently to provide an estimate of the respiratory phase during the acquisition. Spokes were then divided into 4 respiratory frames based on signal amplitude and reconstructed in Matlab using NUFFT algorithms [3]. Motion vector fields (MVFs) between respiratory frames were then estimated using freely-available non-rigid registration algorithms [4].
Cardiac motion was estimated separately using a similar acquisition but with higher resolution of 1.4x1.4x1.4mm and a coronal slab just covering the heart. In addition, imaging proceeded during infusion of 0.2mmol/kg gadolinium-based contrast agent (Multihance, Bracco) to provide contrast between the blood-pool, coronary vessels and myocardium. Respiratory phase was estimated in the same manner before finding the actual head-to-foot displacement from the images. k-Space data for each respiratory phase were phase-shifted to correct for the head-to-foot displacement. Corrected k-space data were then sorted into 3 cardiac frames based on recorded ECG trigger timing (0-300, 300-600, 600-inf ms after trigger) before offline reconstruction. MVFs between cardiac frames were then estimated.
Motion-Phase Monitoring: To employ PET data acquired during the entire 60 minutes, respiratory phase was estimated from the variation of total PET counts over time, similarly to the method used for MR data. ECG trigger times were then used to double-sort all PET data into a matrix of 4 respiratory and 3 cardiac frames.
Motion Corrected PET Reconstruction: Motion correction of PET data employed the reconstruct-transform-average (RTA) approach. Double-gated list-mode PET data were reconstructed offline (e7tools, Siemens) using an iterative algorithm (OP-OSEM, 3 iterations, 21 subsets). Attenuation correction maps [5] of the body were transformed using the estimated respiratory MVFs. Finally, RTA was performed in two steps. All cardiac frames were transformed to the end expiration position using corresponding MVFs. Then each respiratory-motion-corrected cardiac frame was transformed to the diastolic position using the cardiac MVFs before averaging all frames. A flow diagram of the steps involved is given in Fig. 1.
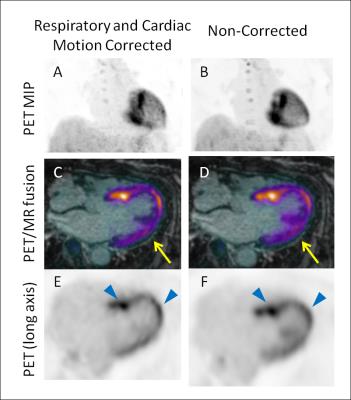
Image analysis: Non-motion corrected (non-MC), and double respiratory and cardiac motion corrected (MC) 18F-FDG-PET images were evaluated qualitatively for image blurring and overall quality and by finding target-to-background ratio (TBR) in cardiac hotspots identified and compared to non-corrected images (blood in the left atrium was used to estimate background).
Results
Focal hotspots of 18F-FDG uptake were observed in the myocardium indicating active cardiac sarcoidosis. Application of the proposed MR-based motion correction scheme showed qualitative improvement in image quality by reducing blurring of the myocardium, particularly in the lateral wall, and by increasing contrast between the hotspots and the diffuse uptake in the rest of the myocardium (Fig. 2). With motion correction, TBR values of the strongest hotspot increased from 5.6 to 5.9.Discussion
We have demonstrated the feasibility of MR-based correction of respiratory and cardiac motion for improved imaging of myocardial PET hotspots in 18F-FDG-PET imaging of patients with cardiac sarcoidosis. These encouraging results warrant further investigation with and optimization of MR-based motion correction for cardiac PET/MR imaging.Acknowledgements
This work was supported by NIH grant R01 HL071021References
[1] Abgral R et al. JACC Cardiovasc Imaging. 2016 Jun 29. pii: S1936-878X. [2] Grimm R et al. Med Image Comput Comput Assist Interv 2013;16:17-24. [3] Fessler J et al. Image reconstruction toolbox, University of Michigan (https://web.eecs.umich.edu/~fessler/code/index.html). [4] Buerger C et al. Medical Image Analysis, 15:551-564, 2011. [5] Robson PM et al. Proc ISMRM 2016 p2545Figures