3897
PET-MR Motion Correction of Entire Listmode Data Sets Using Pilot Tone Navigation1Siemens Healthcare GmbH, Erlangen, Germany, 2Radiology, Center for Advanced Imaging Innovation and Research (CAI2R), New York University Langone Medical Center, New York, NY
Synopsis
The introduction of simultaneous PET-MR provides new opportunities for motion tracking and correction during PET imaging. The ideal clinical workflow for a PET-MR exam would allow for PET data to be continuously acquired alongside any desired MR sequences and to reconstruct a motion-corrected PET image utilizing the entire dataset. In this work we combine a surface coil driven by an external signal generator with an MR motion model to navigate PET data during an entire PET-MR exam. We demonstrate the approach on one human subject that underwent a PET-MR exam.
Purpose
The introduction of integrated PET-MR scanners provides the opportunity to correct PET data degraded by motion using high-spatial-resolution, dynamic MR images. Several approaches have been presented to perform efficient PET motion correction during simultaneous PET-MR exams. Most of these approaches, however, are limited to correcting the PET listmode data collected during the dynamic MRI scan. This effectively discards a large portion of the PET data. In this work, we demonstrate a means to motion-correct all PET listmode data acquired during an entire exam with minimal hardware requirements and no modifications to the MR sequences. A pilot tone signal [1,2,3] driven by an external signal generator is used to track the patient’s motion during all MR scans. In combination with a motion tracking sequence, the pilot tone signal is used to correct all acquired PET data. The process of extracting a respiratory gating signal from the pilot tone is demonstrated on three human subjects. One subject was also simultaneously imaged with PET; motion-corrected reconstructions, based on the pilot-tone gating signal, are shown for this subject.Methods
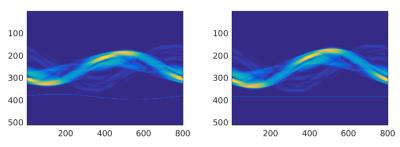
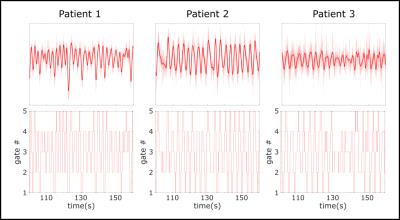
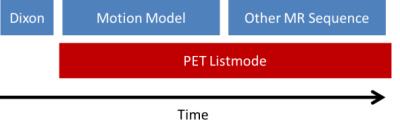
All data sets (N=3) were acquired on a Siemens Biograph mMR (Siemens Healthcare, Erlangen, Germany) using the body coil array in combination with the spine coil and a 2cm surface coil driven by an external signal generator. Data were acquired using an in-house sequence (TR/TE=12.7/5ms, BW=488Hz/Pixel, 80x4.5mm slices, 800 radial views) that supports conventional k-space center self-navigation [4].The sequence also provides motion fields and hence a complete motion model. The coil was mounted on the wall of the scanner room and its frequency was adjusted to guarantee that the generated signal was inside the received frequency band of the scanner but outside the actual object (Figure 1). This enables simple detection and blanking of the pilot signal from the acquired MR images. Two scans were performed for 6 minutes each while PET data were acquired simultaneously for the whole time (Figure 3). In addition, a Dixon sequence was used to acquire data for PET attenuation and scatter correction. Temporal variations of the pilot tone signal represent movement of the patient (Figure 2) and can therefore be used to sort MR and PET data into different motion states. The pilot tone signal was extracted from the second MR data set and synchronized with the motion model. The corresponding motion fields of the motion model were used to correct PET data that were acquired during both MR scans.Results
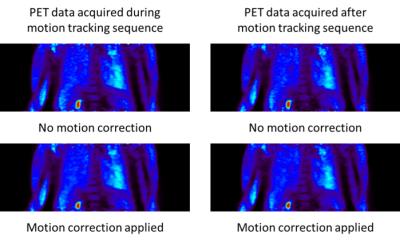
Figure 1 shows MR raw data acquired while using the pilot tone signal to monitor the motion throughout the scan. The pilot tone signal is easy to identify outside the actual object. Synchronization of the pilot tone signal with the motion model was successful as can be seen in Figure 4. The motion-corrected PET images for data acquired during the different MR sequences show the same improvement compared to the uncorrected reconstructions. In both cases the image degradation due to motion blur is reduced significantly.Discussion
The clear separation of the pilot tone signal and signal generated by the sequence allows a simple processing of both signal types. The image quality of the actual MR reconstructions is not biased and the pilot tone signal can be used for the PET navigation. PET motion correction results for PET data acquired simultaneously with the motion model are comparable with results for PET data acquired during other MR sequences. Extending this technique to arbitrary MR sequences is straight-forward because the pilot tone detection step requires only simple filtering operations on the raw data prior to reconstruction.Conclusion
We have demonstrated the possibility of utilizing a pilot tone signal to motion correct PET data from an entire simultaneous PET-MR exam. The presented results relied on a prototype sequence for motion detection but can be combined with any sequence that can provide a motion model. All other MR sequences during the exam do not require any modifications besides a filter applied during data preprocessing to obtain the gating signal and to remove the pilot tone data before the corresponding reconstructions. The flexibility to use the pilot tone with arbitrary sequences combined with the minimal hardware requirements makes the pilot tone a promising tool for respiratory motion correction in PET-MR. Applications to cardiac motion correction are under investigation.Acknowledgements
No acknowledgement found.References
[1] P. Speier et al., Proc. ESMRMB 2015, 129: 97-98.
[2] L. Schroeder et al., ISMRM 2016, #410.
[3] T. Koesters et al., ISMRM 2016, #4250.
[4] R. Grimm et al., Med. Image Anal, 2015, 19: 110-20.
Figures