3886
Rapid Dual Echo UTE MR-Based Attenuation Correction1Department of Radiology, University of Wisconsin, Madison, WI, United States
Synopsis
Recently, ultrashort TE imaging based MR-based attenuation correction (MRAC) has been proposed in literature to overcome the intrinsic difficulty in MRI to resolve bone contrast and hence enable more reliable estimation of attenuation map. However, the long acquisition time required for UTE imaging still remains challenging. In this study, we propose a novel, rapid dual echo method for UTE based MRAC, which allows segmentation of bone, air, fat, and water with high spatial resolution (1mm3) in a single scan with extremely short scan time (35sec).
Purpose
Simultaneous PET/MR has been recently developed to complement each individual imaging modality and obtain molecular-specifc contrast with PET along with the rich soft tissue contrast of MRI. Unfortunately, MR-based attenuation correction (MRAC) remains challenging problem in simultaneous PET/MR imaging due to the intrinsic difficulty in MRI to resolve bone. Ultra-short/Zero TE (UTE/ZTE) imaging-based MRAC acquisitions have been proposed to directly detect signal from short T2* species such as bone1. However, UTE imaging itself typically imposes a long acquisition time (typically 2~5 mins) in addition to the scan time required for fat-water imaging (DIXON or IDEAL). Such acquisitions are not clinically feasible in whole body PET/MR imaging where other imaging sequences in addition to MRAC need to be performed at each PET station. In this study, we propose a novel, rapid dual echo method for UTE based MRAC, which allows segmentation of bone, air, fat, and water with high spatial resolution (1mm3) in a single scan with extremely short scan time (35sec).Methods
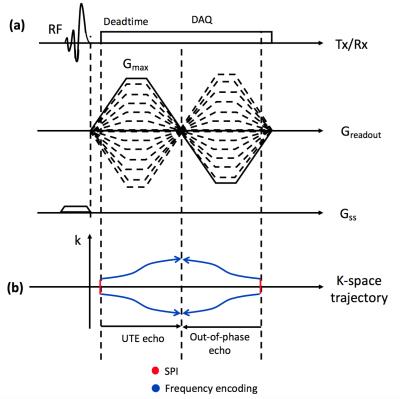
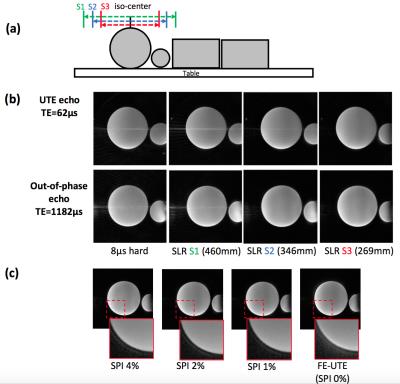
The proposed method utilizes the ramped hybrid encoding (RHE) imaging scheme2 to acquire a UTE image with minimized readout duration to reduce blurriness of short T2* species (e.g., bone). The original RHE sequence was modified to incorporate a SLR half pulse to enable slab selection as shown in Figure 1-a. An out-of-phase echo is acquired after the UTE echo, where data acquired in flying back to the center of k-space are utilized to further shorten the sequence length as shown in Figure 1-b. k-Space trajectory is measured by SPI-based gradient measurement technique3. From the reconstructed UTE (in-phase) and out-of-phase echo images, fat and water separated images are obtained based on a 2-point Dixon reconstruction using the GE Healthcare Orchestra SDK. With the resultant UTE, water, and fat images, a pseudo CT image is synthesized. A soft tissue image is estimated using a water fraction image, while bone and air maps are determined by utilizing inverse log contrast of UTE image. DC bias over the UTE image is corrected ahead of bone and air segmentation using ROI based intensity correction (21x21x21 surrounding pixel was used as ROI). The initial segmentation maps for bone and air are refined using morphologic image processing to remove noise and false detection. Phantom and in vivo imaging were performed using a 40-channel HNU coil in a 3T Signa PET/MR system (GE Healthcare, Waukesha, WI, USA), with the following parameters: Gmax=33mT/m, slewrate=118mT/m/ms, FOV=300mm3, voxel size=1mm3, TR/TEs/scantime=4.2ms/62µs/1.17ms/35sec, FA=1o, # of radial spokes=7442, # of SPI encoding=925. A phantom experiment was performed using water phantoms placed on table as shown in Figure 2-a. In vivo experiment, a head of a human subject was scanned.Results and Discussion
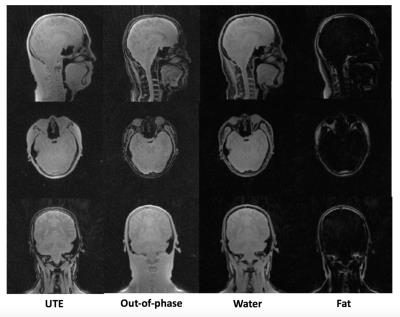
Figure 2-b shows that aliasing/streaking artifact in the S-I direction is suppressed by utilizing slab selection. The image using 8µs hard pulse or larger slab selection exhibits stronger streaking artifact due to the strong readout gradients utilized and the undersampled number of radial spokes necessary for fast imaging. Therefore, it is appropriate to use a selective SLR half pulse with small slab matched the S-I coverage of the PET detector (~25cm). Figure 2-c shows the efficacy of hybrid encoding in reducing the ringing artifact in the image acquired during the flying-back echo, owing to SPI encoded central k-space that is more robust to error in k-space trajectory than frequency encoding based UTE (FE-UTE). Figure 3 shows UTE/In-phase/water/fat images obtained by RHE for the in vivo experiment. Figure 4-a shows DC-biased image, estimated bias map, bias corrected images, and the resultant histogram, used as reference for setting thresholds for bone and air segmentation. Thresholds for bone/air segmentation were set to 1.0/2.2. Figure 4-b shows the estimated CT map, which shows better detection of bone and air compared with system default MRAC provided by GE Healthcare.Conclusion
In this study we have proposed a rapid RHE-based MRAC method that benefits from hybrid encoding and 2-point Dixon encoding. The utilization of a slice selective 3D RHE acquisition improves image quality, while still allowing fast image acquisition. While is also possible to accelerate MRAC by reducing voxel size, the partial volume complicates image-based segmentation techniques. Imaging with a flying-back echo is usually considered to be technically demanding in radial sampling and is not routinely performed. However, with the use of the RHE acquisition (hybrid encoding of central k-space) and robust gradient waveform measurement, it was possible to acquire an out-of-echo in good imaging quality. Such acquisitions are expected to be highly useful for MRAC acquisitions to improve quantitative accuracy in PET/MR.Acknowledgements
We acknowledge support from NIH EB013770 and GE Healthcare.References
1. Wiesinger F, Sacolick LI, Menini A, Kaushik SS, Ahn S, Veit-haibach P, Delso G, Shanbhag DD. Zero TE MR Bone Imaging in the Head. Magn. Reson. Med. 2015;00:n/a–n/a. doi: 10.1002/mrm.25545.
2. Jang H, Wiens CN, McMillan AB. Ramped hybrid encoding for improved ultrashort echo time imaging. Magn. Reson. Med. 2016;76:814–825. doi: 10.1002/mrm.25977.
3. Jang H, McMillan AB. A rapid and robust gradient measurement technique using dynamic single-point imaging. Magn. Reson. Med. 2016;00. doi: 10.1002/mrm.26481.
Figures