3884
Whole-body morphological and functional atlas using an integrated PET-MRI system and fat-water registration1Division of Radiology, Department of Surgical Sciences, Uppsala University, Uppsala, Sweden, 2Centre for Image Analysis, Uppsala University, Uppsala, Sweden, 3Department of Medical Sciences, Uppsala University, Uppsala, Sweden, 4Antaros Medical, Sweden
Synopsis
Today, when whole-body PET-MRI datasets are analyzed, the data is typically reduced to a few a priori specified measurements. New tools are developed in an attempt to utilize the full potential of large whole-body datasets. The purpose of this work was to build a preliminary whole-body atlas, containing both morphological (fat and water MR) and functional (FDG-PET) information on normality. An atlas was built out of 30 subjects using an integrated PET-MRI system together with an efficient registration method for fat-water MR images. The atlas was used in a proof-of-concept anomaly detection in FDG-PET using a pointwise t-test, which successfully detected anomalies in our test subject.
Purpose
Whole-body PET-MRI datasets contain huge amounts of spatially detailed morphological and functional information. Today, when analyzed, these detailed datasets are typically heavily reduced to a few a priori specified measurements of interest and/or visually assessed by a human operator.
This reduction is a limitation especially in systemic diseases such as diabetes and cardiovascular diseases. New tools for large scale image analysis, such as Imiomics1, are emerging in an attempt to utilize the full potential of these whole-body datasets. Statistical knowledge of normality enables new analysis approaches, including anomaly detection and group comparisons.
The aim of this work was to build a preliminary statistical whole-body atlas, with morphological and functional information on normality, and to apply the atlas in proof-of-concept anomaly detection. We used an integrated PET-MRI system together with an efficient registration method for fat-water MR images.
Method
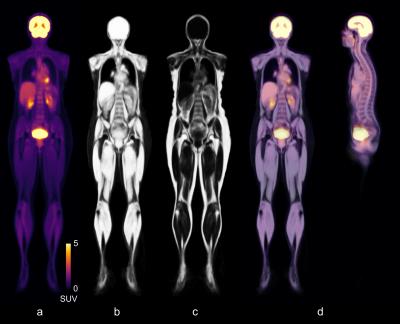
Whole-body images from 31 subjects were used, mean age 67 (±4). The atlas was built by combining 30 healthy subjects from the cohort. Informed written consent was obtained from all subjects (local ethics committee approved).
Images were acquired using an integrated 3T PET-MRI system (Signa PET/MR, GE Healthcare). Fat and water MR images were collected using a Dixon MR Attenuation Correction (MRAC) sequence (TE 1.67ms, TR 4.05ms, voxel size: 2x2x5.2mm). Subjects underwent a PET scan after the injection of [18F]-FDG (2 MBq/kg) with 3 minutes per bed position. The interval between injection and scan start ranged from 100 to 120 minutes. PET reconstruction was performed using GE’s fully 3D Time-of-Flight iterative reconstruction (2 iterations, 28 subsets, standard 5mm filter, and a voxel size of 3.1x3.1x2.8).
Body masks for all subjects were created by using spatial fuzzy C-means clustering (similar to a previously published method2) on intensity inhomogeneity corrected MRAC data. Water- and fat fraction images were generated. In this preliminary work the arms of all subjects were removed and not considered in later analysis steps due to differences in arm positions. All FDG-PET images were converted to Standardized Uptake Values (SUV).
For spatial normalization, a subject representing the cohort average was selected based on BMI and visual assessment. The remaining subjects were registered to the chosen average subject using a fat-water volume image registration pipeline (presented in detail and evaluated elsewhere3):
1. Bone segments (skull, spine, hip, and legs) were segmented for each subject and registered against the chosen average subject using a piece-wise affine registration.
2. The water fraction images and generated masks were registered simultaneously with high spatial regularization to compensate for the low elasticity of muscle tissue. Here the result from (1) was used to fix bone segments.
3. Low regularization registration of fat fraction images with larger water regions fixed using the results from (2).
The obtained deformation fields were used to deform all images to a common space. The atlas was built by computing the mean of all deformed FDG-PET and fat-water images.
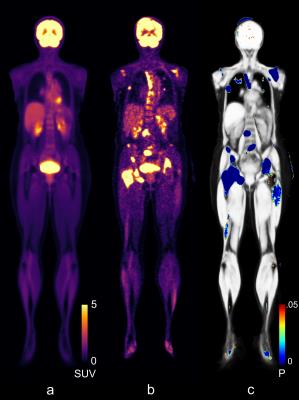
As a proof-of-concept the atlas was used for anomaly detection using FDG-PET data. A subject with reported incidental findings was used for this purpose. The subject was filtered using a Gaussian filter with σ=2.0 and compared to the FDG-PET atlas on a voxel-by-voxel basis, using a two sample, two-tailed t-test.
Results
Figure 1 illustrates the atlas, representing the mean subject of the cohort, built by combining n=30 male and female subjects, by showing the per-voxel mean of all the transformed FDG-PET and water fraction images. For the FDG-PET atlas, there is as expected a high uptake of tracer in the brain, kidneys, and bladder.
The results from the anomaly detection, see Figure 2, agreed with areas containing abnormal uptakes in our subject with incidental findings.
Discussion
The presented method was able to accurately register the whole-body fat-water datasets and create a whole-body atlas for both fat-water data and FDG-PET data. The proof-of-concept showed that a simple t-test can be used to perform voxel-wise detection of anomalies.
Future work includes correction for multiple tests and a more in-depth look into the capabilities of an atlas constructed as presented, e.g. determining the accuracy of the anomaly detection.
Conclusion
We have presented a method for constructing a morphological and functional atlas by using an integrated PET-MRI system together with an MRI fat-water volume image registration method. A proof-of-concept showed the potential for anomaly detection. The method is not restricted to clinical practice as it also allows for holistic analysis which can be used in research studies (e.g. group studies or longitudinal studies). The combination of morphology and functional data allows for a wide range of analysis applications in systemic diseases.
Acknowledgements
Funding was received from the Swedish Research Council (2012-2330) and a research grant from Antaros Medical.References
1. Kullberg J, Johansson L, Lind L, et al. Imiomics: Bringing –omics to whole body imaging: Examples in cross sectional interaction between whole-body MRI and non-imaging data. In Proceedings of the 10th Annual Meeting of ISMRM, Toronto, Canada, 2015. Abstract 3431.
2. Chuang KS, Tzeng HL, Chen S, et al. Fuzzy c-means clustering with spatial information for image segmentation. Computerized medical imaging and graphics 30.1 (2006): 9-15.
3. Ekström S, Malmberg F, Ahlström H, et al. Deformable Registration of Whole-Body Fat-Water Magnetic Resonance Images Using Discrete Optimization. Submitted 2016
Figures