3883
Validation of an Image Derived Input Function Method for PET/MR Brain Scans1Applied Science Lab, GE Healthcare, Menlo Park, CA, United States, 2Applied Science Lab, GE Healthcare, Uppsala, Sweden, 3Nuclear Medicine & PET, Department of Surgical Sciences, Uppsala University, Uppsala, Sweden, 4Radiology Department, Stanford University, Palo Alto, CA, United States
Synopsis
Accurate measurement of the arterial input function (AIF) is essential in quantitative analysis of cerebral blood flow (CBF) using 15O-water PET imaging. The time-of-flight enabled SIGNA PET/MR scanner (GE Healthcare, Waukesha, WI, USA) provides high quality PET images, which can be used for non-invasive Image Derived Input Function (IDIF) estimation. AIF was measured using a proposed IDIF method on 4 patients and the results were compared with the gold standard, arterial blood sampling. The comparison shows excellent correspondence between IDIF and blood sampling, thus validating the IDIF method.
Purpose
Quantification of cerebral blood flow (CBF) is important in the assessment of brain disorders such as stroke or Alzheimer’s disease1. 15O-water PET imaging is an accurate method to measure CBF but it requires the measurement of an arterial input function (AIF), which is usually acquired using invasive arterial blood sampling. A non-invasive Image Derived Input Function (IDIF) method was recently introduced for AIF measurement on a PET/MR scanner2, which estimated spill-over and spill-in artifacts using a PET angiogram (PETA) along with an MR angiogram. Here we have compared the AIF estimation using the proposed IDIF method to the gold standard, arterial blood sampling.
Theory
The ToF SIGNA PET/MR scanner (GE Healthcare, Waukesha, WI) has enough sensitivity to provide quality PET images over a few seconds of 15O-water PET imaging. Using an appropriate time window during the 15O arrival, the cervical arteries can be identified and segmented (PETA)2. These PETA images were used to estimate the spill-over and spill-in artifacts2. An MR phase contrast series was used to image the vessels in the cervical region. These images were used to segment the corresponding cervical arteries in PETA images and the precise volume of those arteries was measured. The total counts from the cervical arteries, after taking the spill-over and spill-in into the account, were divided by the true volume of those arteries (measured from the MR images) to estimate the AIF.Methods
The study was performed in compliance with regulations of the local IRB in Uppsala and all subjects were consented prior to the study. Four subjects (31-50 years old) were injected with 408±62 MBq of 15O-water simultaneously with the start of a 10 min PET scan on the SIGNA PET-MR. During PET scanning, a sagittal vascular (inhance 3D velocity) MR series was used with the following parameters: TR=8.7 ms, TE=4.1 ms, FOV=24×21.6 cm, slice thickness=3 mm, 32 slices, velocity encoding=40, phase acceleration=2.0, and scan time=1:21 min. The PET list file was unlisted for every second and total true and scatter coincident events were plotted to identify tracer arrival into the brain arteries2. Then, a short time frame over the arrival of the tracer to the cervical region was reconstructed to obtain a PET angiogram. The cervical arteries were then segmented using the MR vascular images and PETA images. Spill-over and spill-in artifacts were estimated using PETA images and the actual arterial volume was measured from the MR vascular images. The PET list file was unlisted and images were reconstructed for every 1 s (using a 3 s sliding window) for the first minute, every 5 s for the 2nd minute, every 10 s for the 3rd and 4th minute and every 30 s for 5th to 10th minutes. The AIF was estimated by dividing total counts from the cervical arteries of each frame by the MR-based arterial volume. For each patient, blood samples were continuously drawn from the radial artery at the wrist using a peristaltic pump, and the tracer concentration in the arterial blood was measured using a Twilite two detector (Swisstrace) to estimate the AIF. In order to calculate the AIF at the brain arteries from these blood samples, the delay and dispersion of the arterial input function was corrected using standard PET-based methods3.Results
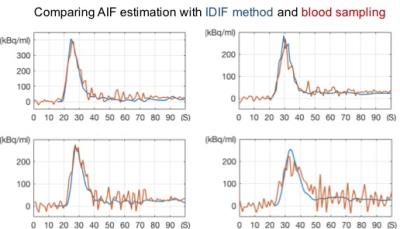
Figure 1 shows the PETA and MR vascular images for one of the patients. The PETA images clearly show the arteries and the extent of the spill-over. Figure 2 shows the cervical arteries mask from PETA and MR images used to estimate spill-over and spill-in artifacts. These masks were used to measure the AIF from the dynamic PET images. Figure 3 compares the AIF curve estimated by the proposed IDIF method2 and the AIF curve measured by the blood samples3. The comparison shows excellent correspondence between the IDIF method and the gold standard blood sampling method with 9% and 11% difference for the 1st pass and the entire AIF, respectively. The IDIF captures the AIF peak correctly and has increased signal-to-noise ratio compared to the blood sampling method. The delay and the dispersion of the AIF curve is nearly identical between the two methods.
Discussion
As the results show, the proposed method is capable of determining a high-fidelity IDIF from simultaneous PET/MRI data. Having a “blood-free” method that obviates the need for direct arterial sampling is of benefit to both investigators and subjects, because of the high costs, inconvenience, and potential risks associated with arterial cannulation. It has applications beyond 15O-water PET, enabling pharmacokinetic modeling to be performed that is required for quantitative PET tracer studies.Acknowledgements
GE Healthcare, Stanford University Lucas Center, Uppsala University.References
[1] Alsop DC, Detre JB. Perfusion magnetic resonance imaging with continuous arterial spin labeling: methods and clinical applications in the central nervous system. Eur J. of Radiology 1999; 30:115-124.
[2] Khalighi MM, Fan A, Delso G, Gulaka P, Shen B, Hoehne A, Singh P, Park JH, Holley D, Chin F, Zaharchuk G. Non-Invasive Estimation of Arterial Input Function for Imaging of Cerebral Blood Flow on a PET/MR Scanner, ISMRM 2016, p4321.
[3] Meyer E. Simultaneous correction for tracer arrival delay and dispersion in CBF measurements by the H2 15O autoradiographic method and dynamic PET. J Nucl Med. 1989; 30:1069-1078.
Figures