3880
Fully-Integrated 3D High-Resolution Multi-Contrast Abdominal PET-MR with High Scan Efficiency1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 2Division of Imaging Sciences and Biomedical Engineering, King’s College London, London, United Kingdom, 3MR Research Collaborations, Siemens Healthcare, Frimley, United Kingdom, 4MR Oncology Application Development, Siemens Healthcare, Erlangen, Germany
Synopsis
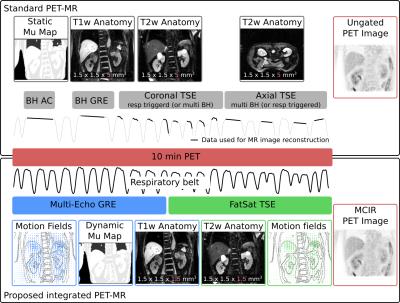
Abdominal PET-MR scans commonly combine free-breathing PET with breathhold or respiratory-triggered MR scans (T1/T2-weighted). This ensures high MR image quality but can lead to PET images impaired by motion blurring. Furthermore, PET can suffer from artefacts close to tissue-lung interfaces due to misalignment of breathhold MR-based attenuation correction (AC) information used for free-breathing PET. Here we present a free-breathing MR-technique which yields motion-compensated 3D T1-weighted and T2-weighted MR images. Respiratory-resolved AC maps and motion compensation improved uptake values (125±131%) and resolution (22±16%). Respiratory motion information is obtained with an accuracy of 1.3±0.1mm without an increase in scan time.
INTRODUCTION
Abdominal PET-MR scans are commonly carried out as a combination of free-breathing PET and breathhold or respiratory-triggered T1-weighted (T1w) and T2-weighed (T2w) MR scans1. Respiratory motion can lead to blurring of the reconstructed PET emission image and can cause misalignment errors between emission and attenuation correction (AC) information (“banana-artefact”)2. Especially when using a breathhold MR-based AC map for the free-breathing PET data, these errors can be substantial. Further misalignment between anatomical MR images acquired in end-expiration and free-breathing PET images can impair diagnostic accuracy. Here we present a technique, which obtains 3D high-resolution T1-weighted Dixon-GRE and 3D high-resolution T2-weighted TSE MR images during a 10min free-breathing scan. Motion-compensated MR and PET image reconstruction (MCIR) is used to minimize respiratory motion artefacts and improve both MR and PET image quality while shortening scan times.METHODS
Free-breathing data acquisition: Eleven patients (10 males and 1 female, 61±11 y, 77±16 kg) were imaged with a prototype T1-weighted 3-point Dixon GRE (FA 12o, TR/TE/DTE 7.3/1.3/1.9) and fat-saturated 3D variable flip angle TSE sequence (FA 90/120°, TR/TE 201/2000ms) on a 3T PET-MR scanner (Biograph mMR, Siemens Healthcare, Erlangen, Germany). The FOV was 288x520x520mm3 with an isotropic resolution of 1.5mm3 leading to a total scan time of 9.3min. PET data was acquired simultaneously 153±23 min after the injection of a PET tracer (10 patients: 337±25 MBq 18F-FDG, 1 patient with neuroendocrine tumors: 169.5 MBq 68Ga-DOTATATE).
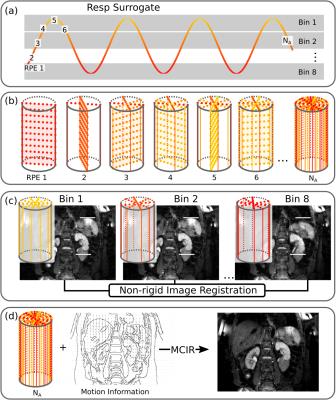
Respiratory motion estimation: The MR data was binned into 8 respiratory motion states based on surrogate information from a respiratory belt (Fig. 2). 3D respiratory-resolved MR images were reconstructed for the T1-weighted and T2-weighted scan3,4. An image registration algorithm was used to obtain non-rigid motion fields describing the movement of each pixel during the respiratory cycle5. The global belt signal ensured that the motion fields transformed the data to the same reference motion state for both T1w and T2w scans.
Motion compensation – MR: The motion information was utilized in MCIR to obtain 3D high-resolution anatomical MR images6.
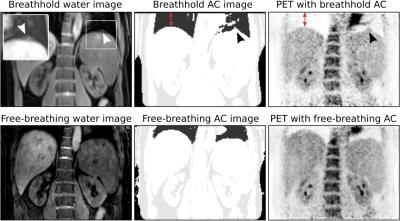
Attenuation correction (AC) map: The MCIR 3-point Dixon GRE scan was separated into fat and water7 and classified as air outside the body (Aout), air inside the body (Ain), fat (F) and soft tissue (S)8. Each tissue type was assigned a constant attenuation value: air 0cm−1, lung tissue 0.02cm−1, soft tissue 0.1cm−1 and fat tissue 0.09cm−1. The motion fields are then used to create a dynamic, respiratory-resolved AC map for the free-breathing PET acquisition.
Motion compensation – PET: Dynamic AC maps and motion information are utilised in a MCIR PET reconstruction (3D OSEM, 23 subsets, 3 iterations, 4 mm isotropic Gaussian post-filtering)9,10. The final spatial resolution of PET was 2.1x2.1x2.0mm3.
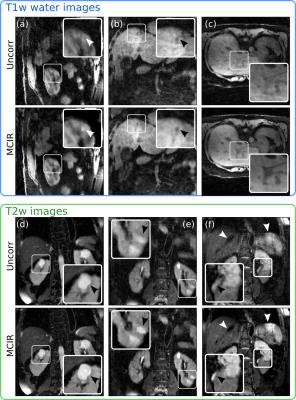
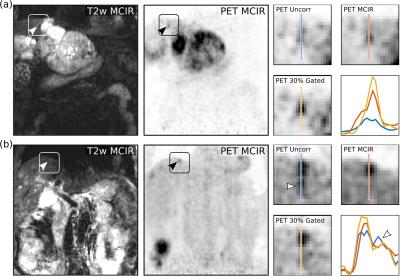
Evaluation: The accuracy of respiratory motion estimation was assessed by comparing anatomical landmark points manually selected in the different respiratory phases to the predictions of the respiratory motion fields (i.e. target registration error). The improvement in MR image quality using MCIR was analysed by calculating the sharpness of the right hemidiaphragm and the edges of the right and left kidney4. The Dice similarity coefficient was calculated between the end-expiratory Dixon-GRPE and standard breathhold MRAC images11. PET image quality was assessed based on the maximum uptake value (SUVmax) and full-width-at-half-maximum (FWHM) of selected tumors.
RESULTS
Respiratory motion led to organ displacements of more than 10mm (mean/std: 6.5±1.6mm) in the abdomen which was captured by the obtained motion fields with an accuracy of 1.3±0.1mm. MCIR improved image quality (Fig. 3) and increased the sharpness of anatomical features by 19±13% compared to the uncorrected MR images in a 54±26% shorter scan time than a comparable gated MR acquisition (5mm gating window). Accurate AC images could be obtained from the MCIR Dixon-GRE scan with Dice coefficients of 1.0±0.001 for Aout, 0.68±0.09 for Ain, 0.72±0.073 for F and 0.81±0.034 for S compared to a standard breathhold MRAC acquisition while avoiding artefacts caused by incomplete or very deep breathholds (Fig. 4). MR-based motion information improved uptake values (125±131%) and resolution (22±16%) of simultaneously acquired PET images (Fig. 5).CONCLUSION
We have presented a 10min free-breathing PET-MR scan which uses MCIR to ensure high MR and PET image quality. The required respiratory motion fields are obtained from anatomical MR scans without an increase in scan time. This approach ensures high scan efficiency and minimizes any misalignment errors between MR and PET, which is especially important for the MR-based AC map.Acknowledgements
No acknowledgement found.References
1.Martinez-Moller A, Eiber M, Nekolla SG, et al. Workflow and Scan Protocol Considerations for Integrated Whole-Body PET/MRI in Oncology. J Nucl Med. 2012;53(9):1415-1426.
2.Sureshbabu W, Mawlawi O. PET/CT imaging artifacts. J Nucl Med Technol. 2005;33(3):156-61-4.
3.Pruessmann KP, Weiger M, Boernert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med. 2001;46(4):638-651.
4.Cruz G, Atkinson D, Buerger C, Schaeffter T, Prieto C. Accelerated motion corrected three-dimensional abdominal MRI using total variation regularized SENSE reconstruction. Magn Reson Med. 2016;75(4):1484-1498.
5.Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18(8):712-721.
6.Batchelor PG, Atkinson D, Irarrazaval P, Hill DLG, Hajnal J, Larkman D. Matrix description of general motion correction applied to multishot images. Magn Reson Med. 2005;54(5):1273-1280.
7.Berglund J, Kullberg J. Three-dimensional water/fat separation and T 2 * estimation based on whole-image optimization-Application in breathhold liver imaging at 1.5 T. Magn Reson Med. 2012;67:1684-1693.
8.Martinez-Möller A, Souvatzoglou M, Delso G, et al. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med. 2009;50(4):520-526.
9.Thielemans K, Tsoumpas C, Mustafovic S, et al. STIR: software for tomographic image reconstruction release 2. Phys Med Biol. 2012;57(4):867-883.
10.Tsoumpas C, Mackewn JE, Halsted P, et al. Simultaneous PET-MR acquisition and MR-derived motion fields for correction of non-rigid motion in PET. Ann Nucl Med. 2010;24(10):745-750.
11.Dice LR. Measures of the Amount of Ecologic Association Between Species. Ecology. 1945;26(3):297-302.
Figures