3874
Accelerated Multicontrast Volumetric Imaging Using Compressed Sensing Parallel Imaging Reconstruction with Low Rank and Spatially Varying Edge-Preserving Constraints: In-Vivo Preclinical Validation for High-Resolution Myocardial Infarction Characterization1Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Schulich Heart Research Program and Physical Sciences Platform, Sunnybrook Research Institute, Toronto, ON, Canada
Synopsis
To improve characterization of myocardial infarction using high-resolution multicontrast volumetric imaging, this work presents a new compressed sensing parallel imaging reconstruction using low rank and spatially varying edge-preserving constraints. The proposed method was validated in vivo in preclinical studies on pigs with chronic myocardial infarction with comparison to histopathology, demonstrating the promise of robust reconstruction of fine image detail from a single breath-hold multicontrast acquisition at an isotropic resolution of 1.5mm.
Background and Purpose
Recent findings from preclinical experiments and clinical patient studies have indicated that an isotropic
resolution on the order of 1.5mm would be appropriate for characterizing the peri-infarct region that might be associated with
ventricular tachycardia1,2,3. Multi-contrast late enhancement (MCLE)4 images offer better visualization of myocardial infarction (MI)
than conventional IR-FGRE. Accelerated three-dimensional (3D) MCLE using a compressed sensing method has been demonstrated in
both preclinical studies5,6 and clinical studies7 for high-resolution MR characterization of peri-infarct regions. However, artifacts in the tissue border area may be present, particularly in images with low contrast-to-noise
ratios (CNR). In this work, a new Compressed sensing Parallel imaging reconstruction with Low rAnk and Spatially varying Edge-preserving constRaints (CP-LASER) was proposed to improve reconstruction for accelerated 3D MCLE with respect to robustness and consistency.Background and Purpose
Theory
Theory
3D MCLE acquires multicoil multicontrast data, where an individual coil dataset is a series of multicontrast volumes acquired at different TIs in the diastolic periods using a balanced SSFP readout after an inversion. CS-LASER6 provides a coil-by-coil reconstruction of multicontrast volumes from a highly accelerated 3D MCLE acquisition using low rank and spatially varying edge- preserving constraints. To take advantage of coil sensitivity information in the CS-LASER framework, a compressed sensing parallel imaging reconstruction can be written as
$$$\hat{\mathbf{C}} = \arg\min_{\substack{\mathbf{C}}} \; \{\sum\limits^{\text{#coil}}\limits_{q=1}\|\mathbf{F_{u}(\mathbf{S}_{q}C\hat{\Phi}})-\mathbf{d}_{q}\|_{2}^{2}+\lambda\sum\limits_{l=1}\limits^{L}J(\mathbf{C}^{(l)})\},$$$
where: $$$\mathbf{d}_{q}$$$ is a vector formed by concatenating the undersampled $$$q$$$th-coil k-space data acquired for each TI; $$$\mathbf{S}_{q}$$$ is a diagonal matrix representing the sensitivity of the $$$q$$$th coil; rows of the pre-estimated $$$\hat{\Phi}$$$ and columns of $$$\mathbf{C}$$$ span the temporal and spatial subspaces of the underlying multicontrast volume series respectively; the operator $$$\mathbf{F_{u}}$$$ performs a partial Fourier transform on each contrast-weighted volume represented by a column of $$$\mathbf{S}_{q}\mathbf{C}\hat{\Phi}$$$ and then concatenates the results into a vector; $$$\mathbf{C}^{(l)}$$$ is a 3D matrix formed by reshaping the $$$l$$$th column of $$$\mathbf{C}$$$; the spatially varying edge-preserving constraint $$$J(\cdot)$$$ is formulated using the weighed total variation, as defined in CS-LASER6; and $$$\lambda$$$ is the regularization parameter. The sensitivity maps $$$\{\mathbf{S}_{q}\}$$$ are estimated using an eigenvalue-based method, referred to as ESPIRiT8. CP-LASER also uses the multiscale iterative reconstruction framework6. The underlying multicontrast volume series can then be obtained as the matrix product $$$\hat{\mathbf{C}}\hat{\Phi}$$$.
Methods
Three Yorkshire pigs with six-week-old infarcts were imaged after injection of 0.2mmol/kg Gadolinium-DTPA using an
ECG-gated 3D MCLE sequence with a 160x160x10 acquisition matrix over a 1.5cm-thick slab with corresponding resolution of 1.5mm3.
The undersampled datasets were prospectively acquired at acceleration rates of 3 and 5 using Variable Density Poisson-disk Sampling
patterns with a 16-channel anterior cardiac coil array in a GE 3T scanner. The 3D MCLE acquisition with 3-fold acceleration was
performed in a navigator-gated free-breathing scan and the 5-fold acceleration was performed in a single breath-hold. IR-FGRE images
were also acquired with the parameters as follows: acquisition matrix = 160x160; FOV = 24cm; slice thickness = 5mm. For comparison, CS-LASER and L1-ESPIRiT8 with wavelet regularization were also implemented. For CP-LASER and L1-ESPIRiT, the multicoil data
were compressed to seven virtual channels9 and used to estimate ESPIRiT coil sensitivity maps. At each scale level for CS-LASER,
a coil-by-coil reconstruction was performed and the weights were then re-estimated from the combined multicontrast volumes using
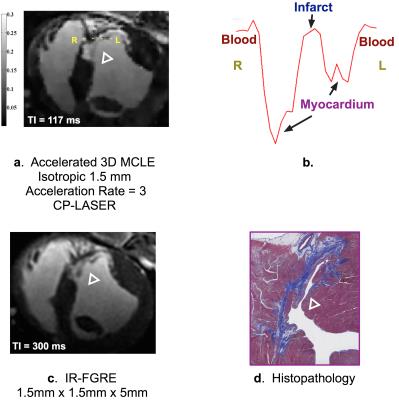
the sum-of-squares method. For the histological processing, representative slices were stained with Masson’s Trichrome, depicting collagen deposition in blue and
healthy myocardium in red. Methods
Results
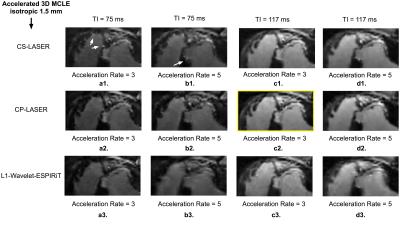
In FIG. 2, CP-LASER provides the best image quality among various methods at acceleration rates of both 3 and 5 for
different TIs; the infarct characteristics from CP-LASER match the best with the findings from the histopathology (FIG. 1d); results
from CS-LASER present artificial edges in the images of the early TI, as indicated by the arrows; L1-ESPIRiT produces results with
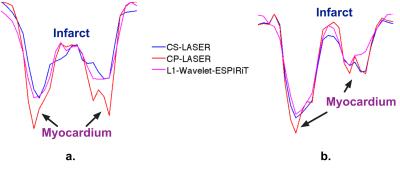
blurry tissue features and residual artifacts. In FIG. 3, CP-LASER presents sharper infarct-myocardium borders than CS-LASER and
L1-ESPIRiT for both the high CNR (FIG. 3b) and low CNR cases (FIG. 3a); and the advantage of CP-LASER is pronounced in the low
CNR case.Results
Conclusions
CP-LASER enables robust reconstruction of fine anatomical detail for highly accelerated multicontrast volumetric
imaging and can provide consistent performance in low CNR situations. We also successfully demonstrated the feasibility of accelerated
3D MCLE with CP-LASER for characterizing the peri-infarct region in vivo at an isotropic high resolution.Conclusions
Acknowledgements
Funding support is acknowledged from GE Healthcare and the Canadian Institutes of Health Research.References
[1] R. Ranjan et. al, Circ Arrhythm Electrophysiol, 2011;4(3):279-86.
[2] R. Ranjan et. al, Circ Arrhythm Electrophysiol, 2012;5:1130-5.
[3] J. Fernandez-Armenta et. al, Circ Arrhythm Electrophysiol, 2013;6(3):528–37.
[4] J. S. Detsky et. al, MRM, 2007;58(2):365-72.
[5] L. Zhang et. al, ISMRM, Singapore, 2016. p.4215.
[6] L. Zhang et. al, MRM, 2016. DOI: 10.1002/mrm.26402.
[7] L. Zhang et. al, ISMRM, Singapore, 2016, p.2542.
[8] M. Uecker et. al, MRM, 2014;71(3):990–1001.
[9] M. Uecker et. al, ISMRM, Utah, USA, 2013, p.2657.
Figures