3849
Improved unbiased multi-slice T1 measurement with compressed-sensing assisted variable-repetition-delay turbo-spin echo acquisition for ultra-high field preclinical applications1Bio-medical Engineering, Ulsan National Institute of Science and Technology, Ulsan, Korea, Republic of
Synopsis
The benefits of compressed-sensing (CS) assisted turbo-spin-echo (TSE), i.e. CS-TSE, acquisition for variable-repetition-delay T1 measurement were investigated with two-dimensional multi-slice ex vivo and in vivo T1 mappings at 7T preclinical scanner. The direct advantages resulting from replacing the refocusing pulses of TSE with CS acceleration included reduced scan times for multi-slice coverage and minimization of inter-slice interferences, which are all required in order to improve the accuracy of multi-slice T1 measurement.
Purpose
To obtain unbiased multi-slice T1 maps for ultra-high field preclinical applications at reasonable scan time by optimizing compressed-sensing assisted variable-repetition-delay turbo-spin-echo acquisition.Methods
The T1 related signal behavior of TSE acquisition1 can be generalized at a certain turbo factor (TF): $$S_{TSE}=S_0(1+\sum_{n=1}^{TF}2(-1)^nexp((-TR+TE(2TF-n+1)/2)/T_1)+2(-1)^{TF-1}exp(-TR/T_1))$$ where S0 is the proton spin density and TE is the echo spacing. The IRSE data were fitted using a conventional signal equation1. Gaussian-weighted (σ2 = 0.125) random undersampling schemes were utilized while maintaining incoherency along the TR direction with the fixed number of phase encodings (~7%) in the low-frequency region of the k-space2. For CS reconstruction, the constrained optimization problem was solved with the sequence of images in the TR direction (s):
minimize$$$\parallel\psi s\parallel_1$$$
subject to$$$\parallel Fs-k\parallel_2<\epsilon$$$
where Ψ denotes the linear operator that transforms s into a sparse representation (2D wavelet and 1D Fourier transforms in the x-y and TR directions, respectively). F is the undersampled Fourier operator, k is the acquired k-space data, and ε controls the fidelity of the CS reconstruction to the measured data, which is usually set to below the noise level of the k-space3,4. The reconstruction was performed using software developed in-house with MATLAB R2015a (MathWorks, Natick, MA, USA). Two external packages were also used: spgl1 v.1.85 and Wavelab v.8.026 for the l1-norm minimization and application of the wavelet transform into images, respectively.
All of the experiments were performed on a Bruker 7T MRI scanner. The Institutional Animal Care and Use Committee of the Ulsan National Institute of Science and Technology approved the animal experiments. A 23-mm transmit/receive volume coil was used in the ex vivo rat kidney experiment. For in vivo abdomen T1 mapping with BL6 mice, a 72-mm transmit volume coil and a mouse brain receive-only surface coil were used.
In order to evaluate the inter-slice interferences between TSE and CS-TSE, multi-slice T1 measurements were performed by varying the slice-gap (0, 100, 200, and 400%). The reference T1 maps were measured with single-slice IRSE acquisitions: ten TIs (ranging from 100 ms to 7500 ms), TR = 7600 ms, TE = 8 ms, matrix size = 256 × 256, slice thickness = 1 mm, and field of view = 16 × 16 and 32 × 32 mm2 for ex vivo and in vivo experiments, respectively.
The TSE and CS-TSE experiments were performed with sixteen logarithmically increasing TRs (ranging from 180 to 7500 ms), an echo spacing of 8.2 ms, a centric echo order, turbo factors = 2 and 8 for the TSEs (TSE2 and TSE8) and turbo factor = 2 for the fourfold undersampled CS-TSE (CS4-TSE2).
In order to compare the in vivo T1 mapping accuracy of CS-TSE with gradient echo based acquisitions, single-slice T1 experiments were performed with TSE2 (for the reference), TSE8, CS4-TSE2, variable-flip-angle spoiled-gradient-echo (VFA-GRE), and Look-Locker inversion-recovery TrueFISP (LL IR-TrueFISP) acquisitions.
Results
Figure 1 shows the inter-slice interferences for the multi-slice ex vivo kidney T1 experiment. In the two-slice experiment, TSE8 resulted in overestimation of T1 values compared with those from corresponding TSE2 and CS4-TSE2 acquisitions. The T1 mapping results of the eight-slice experiment showed the benefit of reduction in scan time from CS undersamplings for extended slice coverage.
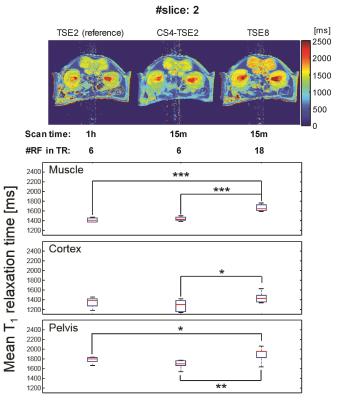
In vivo T1 maps, corresponding ROI-based mean T1 values, and statistical comparisons of back muscle and two regions of the kidney (cortex and pelvis regions) are shown in Figure 2. As in the ex vivo experiment, the mean T1 values of CS4-TSE2 had negligible T1 difference to those of the reference TSE2 acquisition. However, the mean T1 values of TSE8 showed significant differences to those of TSE2 (in the muscle and pelvis regions) and CS4-TSE2 (in the muscle, cortex, and pelvis regions).
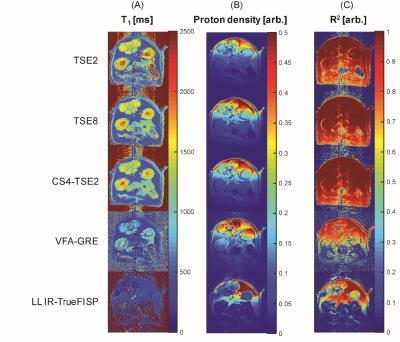
The Figure 3 illustrate the T1 mapping qualities of variable T1 mapping acquisitions. In general, TSE and CS-TSE acquisitions provided more robust measurements over gradient echo based fast T1 acquisition methods in ultra-high field preclinical scanner.
Discussion
CS4-TSE2 acquisition provided consistent T1 measurement accuracy, while TSE8 acquisition manifested overall shift errors in T1 values due to the inter-slice interferences. This is particularly important advantage of CS-TSE over TSE for preclinical applications, because it is difficult to arbitrarily increase slice gap of small animal target tissue.
Limitations of CS-TSE T1 mapping are as follows. First, for in vivo applications, local smoothing artifacts from CS regularizations were apparent. Improvements of CS reconstructions algorithms and the synergistic optimizations with parallel imaging may further reduce in vivo smoothing artifacts at accelerated acquisition time, which may be useful for the region with significant susceptibility artifacts for fast gradient echo acquisitions.
Acknowledgements
This work was supported by National Research Foundation of Korea Grants 2010-0028684 and 2014 R1A1A1 008255, funded by the Korean Government.References
1. Hendrick RE, Raff U. Image Contrast and Noise. DD Stark, WG Bradley (Eds), Magnetic Resonance Imaging (2nd Edition), Mosby-Year Book, St Louis, Missouri 1992:109-144.
2. Efraimidis PS, Spirakis PG. Weighted random sampling with a reservoir. Inform Process Lett 2006;97(5):181-185.
3. Han S, Paulsen JL, Zhu G, Song Y, Chun S, Cho G, Ackerstaff E, Koutcher JA, Cho H. Temporal/spatial resolution improvement of in vivo DCE-MRI with compressed sensing-optimized FLASH. Magnetic resonance imaging 2012;30(6):741-752.
4. Han S, Cho H. Temporal resolution improvement of calibration-free dynamic contrast-enhanced MRI with compressed sensing optimized turbo spin echo: The effects of replacing turbo factor with compressed sensing accelerations. J Magn Reson Imaging 2015.
5. Van Den Berg E, Friedlander MP. Probing the Pareto frontier for basis pursuit solutions. SIAM J Sci Comput 2008;31:890-912. 6. Donoho DL, Stodden VC, Tsaig Y. About sparselab. 2007.
Figures