3844
An optimized slice-GRAPPA reconstruction method to reduce leakage artifacts in small-animal multiband imaging1Center for Information and Neural Networks, National Institute of Information and Communications Technology, Suita, Japan, 2Department of Structural BioImaging, Kumamoto University, Kumamoto, Japan
Synopsis
Accurate slice separation for simultaneous multi-slice acquisition continues to be challenging, especially when animal scanners equipped with relatively few receiver coil elements are used. We propose an optimized slice-GRAPPA method to reconstruct the dual-band EPI of rat brains, in which the size-optimized kernel was iteratively estimated to reduce artifacts. The reconstructed images were evaluated in terms of inter-slice leakage, g-factor, and temporal variation across the repetitions. With the proposed method, inter-slice leakage artifacts and the g-factor were reduced, and the average signal-to-noise ratio was improved. Thus, the total reconstruction accuracy was improved in the multiband EPI in a small animal study.
Introduction
Simultaneous multi-slice (SMS) acquisition can be performed generally well on a recent high-specification MRI system for humans with redundant numbers of receiver coil elements. Accurate slice separation for SMS data thus continues to be challenging, especially when animal scanners equipped with relatively few receiver coil elements are used.In this study, we propose an optimized slice-GRAPPA (SG) reconstruction method for the dual-band EPI of in vivo normal rat brains scanned with an animal scanner having a four-element receiver array coil. This study aims to modify and optimize the SG technique1 and its variant2 for reducing artifacts, such as inter-slice signal leakage, and for maximizing and stabilizing the signal-to-noise ratio (SNR) in the reconstructed images. It also seeks to demonstrate the feasibility of the method through application to dual-band EPI measurements of in vivo rat brains.Methods
Dual-band EPI measurements
In vivo male Wister rat brains (n=8) were scanned on a 7T animal scanner (Bruker, BioSpec70/20) equipped with a transceiver RF coil and a receiver array coil with four elements.A custom-built single-shot dual-band gradient echo 2D EPI sequence was used with blipped-controlled aliasing for the main scans, which were preceded by reference scans with a single-band 2D EPI sequence. Dual-band excitation pulses were designed on the basis of sync functions with frequency offsets, corresponding to a between-band spacing of 9.75 mm. A total of 78 slices (39 dual-band excitations) were imaged in a single repetition. The repetition time and echo time were 2,500 and 15.0 ms, respectively. The voxel size in the plane was 0.25 mm × 0.25 mm, and the slice thickness was 0.25 mm.The encoding and imaging matrix sizes in the read-out and phase-encoding directions were 96 and 56, respectively (rectangular FOV).Second-order shimming within the imaging region was performed prior to EPI acquisition.
EPI image reconstruction method
All the single-band and dual-band EPI raw data were processed with a custom-built reconstruction software program designed for the purpose of this study. The first step in EPI reconstruction was the phase correction between the odd and even lines in k-space. Then, the folded images were separated into each slice at the original location. In this study, a modified SG method was proposed on the basis of the leak-block technique2. We introduced an iterative calculation procedure to estimate and apply the kernels for slice separation. In the iterative SG method, the k-space data for each slice were estimated iteratively until convergence by applying the updated kernels. In the non-iterative SG method, the k-space data for each slice were estimated only once by applying the initial estimates of kernels. Then, in turn, the kernels were updated with the newly estimated k-space data to minimize the leakage artifacts. The estimated kernel size was optimized for better performance. The original SG method1 was also performed for comparison purposes.
Evaluation of the method
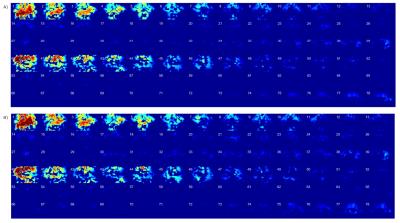
The performance of the reconstruction methods was evaluated in terms of inter-slice leakage and g-factor. Leakage maps3 and g-factor maps were generated for the evaluations. The value of the g-factor was calculated for each slice.
Results and Discussion
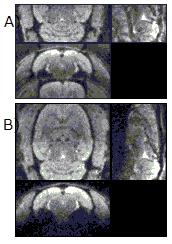
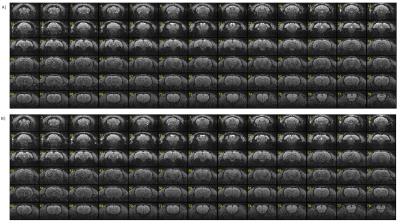
The proposed reconstruction method can provide accurate and stable results in reconstructed images for dual-band EPI scans obtained on a 7T animal scanner (equipped with a 4ch receiver coil). The magnitude images and g-factor maps of all the slices reconstructed with the use of the proposed SG method are shown in comparison with those of conventional SG methods (Fig. 1, 2, 3). The leakage maps of the proposed and the conventional methods are shown in Fig. 4. The proposed iterative kernel computation method for the slice separation was effective in reducing artifacts in the dual-band EPI in our animal scans.Conclusion
An optimized reconstruction method was proposed to reduce leakage artifacts and improve SNR in the dual-band EPI of in vivo normal rat brains. The proposed method allows slice acceleration in animal imaging by improving reconstruction quality, which results in the faster acquisition of whole-brain coverage of small animal brains without sacrificing spatial resolution in the slice-encoding direction (isotropic voxels can be achieved). Furthermore, the proposed method improves reconstruction accuracy in multi-band imaging and thus widens the range of applications of multi-band imaging, such as functional and diffusion MRI, as well as arterial spin labelling. The method can be used not only for slice acceleration in animal imaging but also for a more efficient multi-band EPI in humans, which can be scanned even with a clinical scanner system equipped with relatively few receiver coil elements.Acknowledgements
This study was supported by JSPS KAKENHI Grant Number 25351003.References
1. Setsompop K, Gagoski BA, Polimeni JR, et al. Magn Reson Med (2012).
2. Cauley SF, Polimeni JR, Bhat H, et al. Magn Reson Med 72:93-102 (2014).
3. Moeller S, Xu J, Auerback EJ, et al., Annual meeting of ISMRM, p.519 (2012).
Figures