3840
Snapshot whole-brain T1 mapping using 2D multi-slice variable flip angle spiral imaging with steady-state preparation1Division of Radiological Physics, Department of Radiology, University of Basel Hospital, Basel, Switzerland, 2Department of Biomedical Engineering, University of Basel, Basel, Switzerland, 3Siemens Healthcare, Application Development, Erlangen, Germany
Synopsis
Spiral imaging substantially shortens MR fingerprinting acquisitions to tolerable scan times. Also more traditional “state-of-the-art” parametric mapping techniques can potentially benefit from the speed-up ability of fast spiral trajectories as opposed to Cartesian sampling. Here, a variable flip angle T1 quantification approach based on an interleaved 2D spiral multi-slice spoiled gradient echo sequence is combined with a steady-state preparation scheme. The investigated method offers accurate whole-brain T1 determination at clinically relevant resolution in only half a minute (including the B1 mapping scan in about 40 s) with an acceleration factor of an order of magnitude compared to conventional Cartesian sampling.
Introduction
While spiral imaging has received increased interest in the recent years mainly due to its ability to speed up MR fingerprinting acquisitions, here, it is combined with an interleaved 2D multi-slice spoiled gradient echo (SPGR) sequence and a steady-state preparation scheme to achieve rapid and accurate snapshot-like whole-brain variable flip angle (VFA) T1 mapping at 1.5T.Methods
Imaging protocol. To effectively eliminate the transverse coherences, an interleaved 2D multi-slice SPGR protocol with a long TR of 250 ms (TE = 3.5 ms) and a large total spoiler gradient area within TR of AG = 689 (mT/m)∙ms as recently proposed 1 is used for human brain imaging at 1.5T (Magnetom Avanto fit, Siemens Healthcare, Erlangen, Germany). In total, 55 slices of 3 mm thickness and 1.3 x 1.3 mm2 in-plane resolution are measured in five serial scans; in each acquiring a group of 11 slices (cf. Fig. 1). The measurements are repeated for two flip angles optimized for T1 quantification: α1 = 17° and α2 = 80° 2.
Spiral readout. A dual-density spiral-out trajectory is used in the prototype sequence for k-space sampling 3 and the images are reconstructed with an iterative non-cartesian self-consistent parallel imaging reconstruction (SPIRiT 4). Ten spiral interleaves are acquired (readout time: 11.5 ms, receive bandwidth: 400 Hz/pixel), yielding an acquisition time for one slice group, i.e. 11 slices, of 2.5 s. Due to the short scan times, steady-state preparation is crucial.
Steady-state preparation. Instead of playing a number of “dummy” repetitions, the steady-state is prepared in minimal time based on a single preparation pulse with optimized flip angle β and recovery time Trec (cf. Fig. 1). Optimization of the variables β and Trec is performed by modifying the procedure in Ref. (5) to include the effect of the (non-rectangular) slice profile by a hard-pulse approximation. Optimized parameters found for human brain tissue characterization are: β1 = 34° / Trec,1 = 250 ms (for α1 = 17°) and β2 = 102° / Trec,2 = 350 ms (for α2 = 80°).
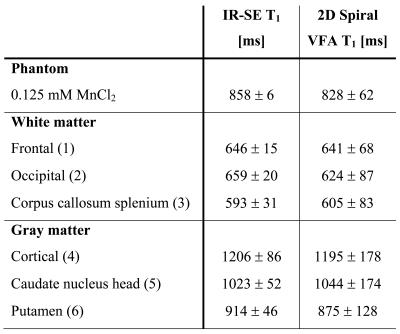
T1 calculation. T1 is quantified based on the Ernst equation describing the ideally spoiled steady-state signal by incorporation of RF field inhomogeneity (obtained from a fast B1 mapping scan of 12 s duration 6) and the slice profile 1,7. The accuracy of the method is assessed both in vitro using a manganese-doped aqueous probe (0.125 mM MnCl2 in H2O) and in vivo for human brain tissues by comparison to a gold standard inversion-recovery spin-echo technique.
Results
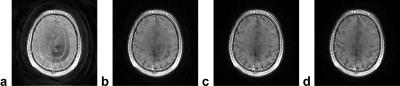
As shown in Fig. 2a for a 2D multi-slice SPGR human brain measurement with a flip angle of 80°, immediate spiral image acquisition without steady-state preparation can lead to artifacts in the high-flip-angle scans related to the transition to steady state (in particular of the cerebrospinal fluid (CSF) due to its long T1 values). The investigated optimized steady-state preparation scheme yields images of good quality in minimal preparation time (cf. Fig. 2d), while the images acquired after one (Fig. 2b) and two (Fig. 2c) ordinary “dummy” repetitions are still prone to steady-state artifacts.
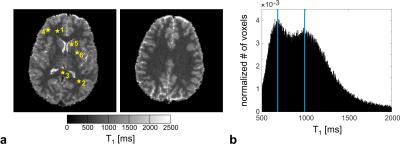
The in vivo T1 maps obtained with spiral 2D multi-slice SPGR imaging and optimized steady-state preparation in the brain of a healthy volunteer at 1.5T appear artifact-free with reasonable signal-to-noise ratio and demonstrate a homogeneous T1 contrast (cf. Fig. 3a). Good agreement with the inversion-recovery reference method is observed both in vitro and in vivo for the selected regions-of-interest indicated in Fig. 3a. Overall, the deviation from the gold standard reference measurement is below 5.5% (cf. Table 1). The whole-brain T1 histogram reveals a clear delineation of the white-matter and gray-matter T1 peaks (cf. Fig. 3b).
Discussion
As evident from the good agreement with the reference method, 2D multi-slice VFA T1 mapping of human brain tissues can be highly accelerated by spiral readouts and steady-state preparation schemes while maintaining its accuracy. The observed lower standard deviations of the reference measurement (cf. Table 1) are simply a result of the long acquisition times and the consequently high SNR (11 min 18 s for a single slice). The total scan time achieved for whole-brain spiral VFA T1 mapping is only about 28 s (by adding the acquisition time for B1 mapping about 40 s). As a result, the presented method provides an acceleration factor of about 10 compared to Cartesian sampling and challenges MR fingerprinting schemes regarding efficiency.Conclusion
The investigated method significantly speeds
up 2D multi-slice VFA T1 quantification and provides snapshot-like
whole-brain T1 mapping at clinically relevant resolution in about half
a minute with high accuracy.Acknowledgements
This work was supported by a grant from the Swiss National Science Foundation (SNF 325230-153332).References
1. Heule R, Bieri O. Rapid and robust variable flip angle T1 mapping using interleaved two-dimensional multislice spoiled gradient echo imaging. Magn Reson Med 2016.
2. Deoni SC, Rutt BK, Peters TM. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magn Reson Med 2003;49(3):515-526.
3. Meyer CH, Zhao L, Lustig M, Jilwan-Nicolas M, Wintermark M, Mugler JP, Epstein FH. Dual-density and parallel spiral ASL for motion artifact reduction. Proceedings ISMRM 2011. p 3986.
4. Lustig M, Pauly JM. SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space. Magn Reson Med 2010;64(2):457-471.
5. Busse RF, Riederer SJ. Steady-state preparation for spoiled gradient echo imaging. Magn Reson Med 2001;45(4):653-661.
6. Chung S, Kim D, Breton E, Axel L. Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout. Magn Reson Med 2010;64(2):439-446.
7. Parker GJ, Barker GJ, Tofts PS. Accurate multislice gradient echo T(1) measurement in the presence of non-ideal RF pulse shape and RF field nonuniformity. Magn Reson Med 2001;45(5):838-845.
Figures
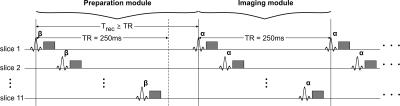
Figure 1. The 2D multi-slice SPGR acquisition consists of a preparation module with a single preparation pulse of optimized flip angle β. After the recovery period Trec (≥ TR), the actual acquisition starts with excitation pulses of flip angle α and a TR of 250 ms. In each TR, 11 slices separated by an edge-to-edge spacing of four times the slice thickness to mitigate slice cross-talk and magnetization-transfer-related issues are acquired interleaved. Five slice groups (of 11 slices each) positioned interleaved with respect to each other are measured in five serial scans yielding in total 55 slices with full-brain coverage.