3837
Super Resolution Motion Correction (SUPREMO) using Simultaneous Multi-Slice EPI based fMRI1Department of Radiology, University of California San Diego, La Jolla, CA, United States, 2Department of Neurosciences, University of California San Diego, La Jolla, CA, United States
Synopsis
In this study, we describe a method to correct for motion in fMRI acquisitions with sub-TR temporal resolution, applying the Extended Kalman Filter framework, using each multi-slice EPI shot as its own navigator.
Purpose
Subject motion is known to produce spurious covariance among time-series in functional connectivity studies1. Spurious developmental resting state fMRI effects due to residual motion have been reported in children2,3. Nearly every functional MRI (fMRI) study implements rigid body realignment, assuming no motion within each TR, and thus cannot correct for intra-TR motion. To reduce contamination due to uncorrected sub-TR motion, a common practice is to censor timepoints with motion above a specified a framewise displacement threshold4. This can result in a severe loss of data, particularly for children, the elderly, and patients populations where motion may be more or less continuous across time. The development of simultaneous multi-slice (SMS) acquisitions allows for the collection of multiple slices within a single echo planar imaging (EPI) readout. Previous work has demonstrated the utility of using the Extended Kalman Filter (EKF) for real-time image-based motion correction with spiral-navigated acquisitions5. The purpose of the current study is to present an initial feasibility study for applying the EKF framework for super temporal resolution motion correction (SUPREMO) using each multi-slice EPI shot as its own navigator (i.e., self-navigated).Methods
Image Acquisition: The study was approved by the local Institutional Review Board (UCSD IRB). Two healthy volunteers were recruited and provided written informed consent. Six resting-state fMRI runs were acquired on a 3T GE DISCOVERY MR750 scanner using a 32-channel head coil with the following parameters: TR/TE (ms)=800/30; in plane resolution=2.4x2.4 cm2; matrix=90x90; number of slices=60; slice thickness=2.4 mm; multiband factor=6; number of temporal frames=380. For 3 runs the volunteers were asked to remain as still as possible, while for the other 3 runs they were asked to rotate their head continuously around the z axis (yaw rotation).
Image Processing: The average volume of the first rsfMRI run without staged motion was employed as the reference volume for motion correction. Motion correction was implemented in two different ways for all fMRI runs:
1. Conventional volume-wise rigid motion estimation using mcflirt (FSL).
2. The proposed framework with self-navigated EKF motion estimation and correction with sparse resampling to high-temporal resolution (SUPREMO). This framework assumes each reconstructed multi-slice image to be a sparse version of a full image volume, allowing for high spatiotemporal volume resampling and motion correction with tunable smoothness parameters minimizing the following cost function
$$arg_{x}min\left\{\parallel Mx-y\parallel^2+\lambda_{s}\parallel R_{i}x\parallel^2+\lambda_{s}\parallel R_{j}x\parallel^2+\lambda_{s}\parallel R_{k}x\parallel^2+\lambda_{t}\parallel R_{t}x\parallel^2 \right\}$$
where $$$x$$$ is a vector form of the full 4-D spatiotemporal motion corrected volume of interest, $$$y$$$ is a vector form of the observed spatiotemporal volume (i.e., acquired images), and $$$M$$$ is a large sparse matrix that resamples $$$x$$$ into $$$y$$$ according to the high-temporal resolution motion estimates. Additionally, $$$R$$$ are matrix derivative operators for the three spatial directions $$$(i,j,k)$$$ and time dimension $$$t$$$, and $$$\lambda_{s}$$$ and $$$\lambda_{t}$$$ are tunable “roughness penalty” parameters, in the spatial directions and time dimension respectively.
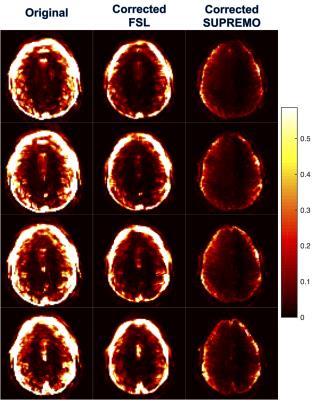
Temporal variance maps, normalized to the image squared mean intensity, were calculated for the original fMRI series, the FSL corrected series, and the corrected series using SUPREMO. Summary statistics of the variance maps were obtained applying a dilated brain mask to the parametric map.
Results
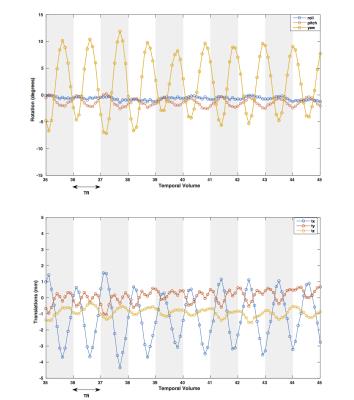
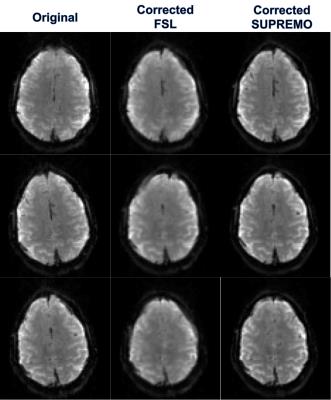
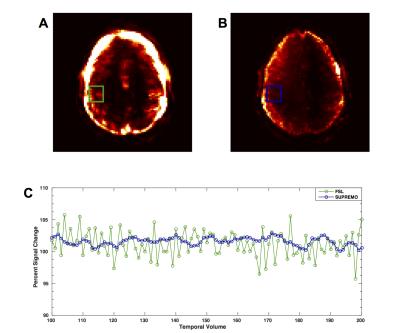
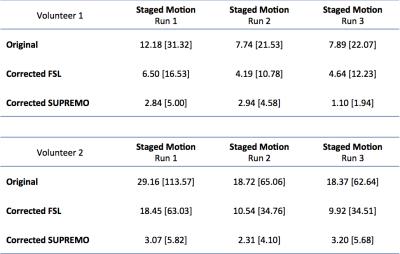
In Figure 1 it is shown how the proposed correction framework successfully estimated sub-TR staged rotations of up to 12 degrees that were not detected by conventional volume-wise motion estimation. Figure 2 shows that even with large rotations within the same volume, the sub-TR motion corrected series are realigned. After correction, the temporal variance was reduced for the staged motion series when compared with the original volumes and the corrected series using volume-wise motion estimation. Normalized temporal variance maps for the original series and both of the correction methods are reported for a series of consecutive slices in Figure 3. Figure 4 shows a reduction in the temporal signal percent change after applying SUPREMO when compared with the same region of the volume-wise motion correction. For all staged-motion runs the variance was reduced when using SUPREMO. The median and interquartile range of the normalized variance maps for both volunteers are reported in the Table 1 for the 3 staged-motion runs performed.Conclusion
Our results demonstrate the feasibility of using simultaneous multi-slice EPI shots as self-navigators to estimate and correct for fast sub-TR motion by assuming each multi-slice shot is a compressed sensing version of a high-temporal resolution complete volume. This development might provide a tool to improve fMRI analysis, in particular in un-sedated children. In addition, the framework proposed can be further extended to account for signal variations due to other factors influencing signal intensity values as coil sensitivity and spin history.Acknowledgements
This work was supported by the National Institutes of Health [U24DA041123], and General Electric [Investigator Initiated Research, Award BOK92322 (N.S.W)].References
1. Power JD, et al. Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage 2015.
2. Van Dijk KRA, et al. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage 2012.
3. Power JD, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 2012. .
4. Power JD, et al. Methods to detect, characterizes, and remove motion artifact in resting state fMRI. NeuroImage 2014.
5. White NS, et al. PROMO: Real-Time Prospective Motion Correction in MRI using image-based tracking. Magn Reson Med 2010.
Figures