3826
An Automated Phantom QA procedure for the Rhineland Study1German Center for Neurodegenerative Diseases, Bonn, Germany, 2Department of Physics and Astronomy, University of Bonn, Bonn, Germany
Synopsis
For the Rhineland study, a fully automated phantom QA procedure was developed to monitor the quality characteristics of the MRI scanners. Various QA metrics are investigated to characterize scanner stability and performance over time and across different sites. To ensure measurement reproducibility and consistency standardized phantoms were developed and a dedicated QA protocol as well as a processing pipeline was implemented. The QA scans are acquired each day after the last subject to be able to detect data inconsistencies and hardware defects as soon as possible. This ensures high-throughput data collection with minimal data loss.
Introduction
The Rhineland Study is a population study and investigates the aging brain longitudinally using MRI. The data are acquired at different sites on identical scanner hard- and software. To ensure high-throughput data collection (150-200 adults weekly) a fully automated quality assurance procedure more than once a week is necessary. Each day after the last subject a phantom QA scanning session is performed. This keeps potential data loss due to unexplained variance in the data (malfunctioning hardware, system upgrades) at a minimum. To be comparable over different sites a standardized and reproducible QA phantom was developed including the phantom mount. Furthermore, a QA protocol based on the Rhineland study protocol was implemented. The data analysis is performed using an in-house developed, automated processing pipeline. Various MR parameters are longitudinally assessed over time and sites.Methods
A phantom consisting of a homogeneous gel mimicking brain gray matter was developed. As phantom housing a glass sphere (18cm diameter) was chosen to achieve a homogeneous main magnetic field. To reproduce the phantom position inside the scanner over sites and sessions, a phantom mount was built.The MR protocol consists of the complete Rhineland study protocol1, matching the in vivo protocol exactly: B0/B1 mapping, 3D-EPI, DTI, QSM, and structural imaging (T1/T2/FLAIR). Additionally, a gradient echo scan (2 measurements for SNR analysis2) is acquired with head and body coil. The gradient echo imaging data is saved uncombined (per receive channel) to get information about the individual receive channels. All imaging was performed on MAGNETOM PRISMA scanners (Siemens, Healthcare, Erlangen, Germany) using a 64-channel head coil. The total MR protocol takes 52min. The resulting data are sent to a XNAT server3 by the technicians. Afterwards, an in-house developed processing pipeline based on Nipype4 is triggered. The analysis is performed automated on compute servers:The data is converted to the Nifti format and the image header information is checked for protocol consistency (TE/TR/FOV, etc...) and system consistency (frequency/RX/TX/gradient adjustments). Afterwards, the actual QA processing on the image data is performed: The EPI data is analyzed employing an fBIRN pipeline5, DTI scans are processed using a DTIFit pipeline6. The additional GRE scans are used to generate recieve sensitivity maps, channel contribution maps, and SNR maps. In each map and imaging result a region of interest in the center of the phantom is drawn and the containing voxels are analyzed. The derived values of the corresponding imaging parameters are compared to reference values in a database. The results are stored in a JSON file on the XNAT system. Values outside their confidence interval are marked. A final report and summary images are stored as well. Finally, after pipeline completion, a visual check of the results is performed by a trained eye.Results
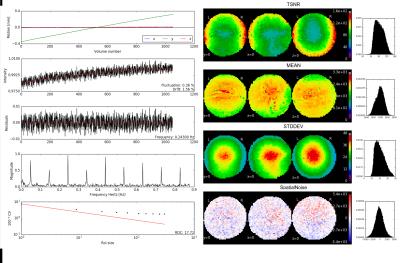
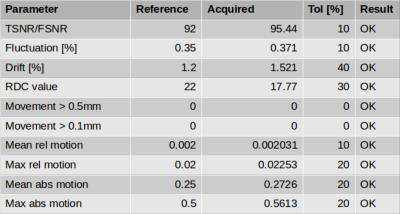
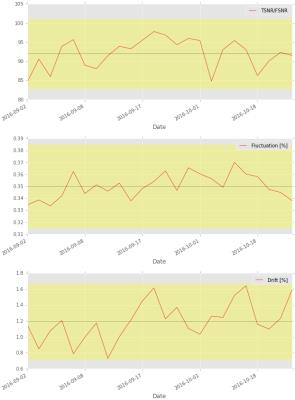
Figure 1 shows the model of the phantom mount and an image of the phantom in the head coil. The mount is MR invisible and provides a high positioning accuracy. An acquisition of the final phantom gel is shown in figure 2. The standardized construction recipe allows to reproduce the phantom gel with high accuracy with an error in the MR parameters of approximately 5%. The gel is long term stable and does not show any signs of molding or degeneration longitudinally. The gel is homogeneously mixed and contains only a few bubbles. Thus, producing a homogeneous main magnetic field. The processing pipeline runs automatic and produces 4.5 GB data/day/scanner. Minimum user interaction is needed (phantom positioning, upload of scans, final visual check) and is below 10 minutes per day.In figure 3 the summary image from the fBIRN pipeline is depicted. In figure 4 the calculated metrics are shown. The time course of the fBIRN parameters is depicted in figure 5.Discussion
Scanner stability is one of the most important factors for longitudinal studies, especially when imaging is performed at multiple sites. The presented procedure allows to monitor all Rhineland-Study MR-systems under the same conditions longitudinally. To create identical scanning conditions as in vivo (system load, gradient heating), the complete Rhineland protocol is acquired. Unexplained variance in the data that may occur only in specific protocol constellations may be better reproduced this way. The QA procedure can be used to evaluate the scanner after installation of faulty components to exclude changes in the system. As already shown in the past5, even without these necessary system modifications, scanners change slightly over the years. Therefore, it becomes potentially necessary in the future to adapt the confidence intervals for the calculated metrics when more data is acquired.Acknowledgements
No acknowledgement found.References
1Breteler et al. Alzheimer's and Dementia. 2014;10(4):92
2Dietrich et al. J Magn Reson Imaging. 2007;26(2):375-85
3Marcus et al. Neuroinformatics. 2007;5(1):11–34.
4Gorgolewski et al. Front Neuroinform. 2011;5:13
5Friedman L, Glover GH. J Magn Reson Imaging. 2006;23(6):827-39
6Jenkinson et al. Neuroimage. 2012;62,782-790
Figures