3823
Hough-transform based detection of vascular structures applied to automate and accelerate planning of super-selective Arterial Spin Labeling1Clinic for Radiology and Neuroradiology, University Hospital Schleswig-Holstein, Kiel, Germany, 2Tomographic Imaging Department, Philips Research, Hamburg, Germany
Synopsis
Super-selective Arterial Spin Labeling (ASL) is a technique to perform non-contrast enhanced flow territory mapping. Prior to image acquisition, the labeling focus has to be positioned on each artery of interest separately. Depending on the arterial architecture, this process can be time-consuming, especially for untrained operators. In this study, an algorithm for automated vessel detection and planning is introduced to accelerate the planning procedure of super-selective ASL measurements, which is based on the Hough transform to detect circular structures (i.e. arteries) on a transversal time-of-flight (TOF) scan.
Introduction
Arterial Spin Labeling (ASL) is an established technique to perform non-contrast enhanced MR perfusion imaging. Modifications of the labeling plane allow for the selective visualization of individual brain feeding arteries, mostly referred to as the internal carotid arteries (ICAs) and vertebral arteries (VAs). To acquire super-selective pCASL, the labeling focus has to be positioned on each artery of interest separately [1]. This process of identifying the artery of interest and placing the labeling focus (shifting and tilting) is -especially for untrained users- more time-consuming than conventional (non-selective) ASL imaging. In this study, an algorithm for automated vessel detection and planning is introduced to accelerate the planning procedure of super-selective ASL measurements. This approach is based on the Hough transform to detect circular structures (i.e. arteries) on a transversal time-of-flight (TOF) scan [2].Materials and Methods
The algorithm was implemented in Matlab R2013b (The Mathworks, Natick, MA) using the image processing toolbox. On each slice of the acquired TOF image, the Hough transformation to detect circular objects is applied. The user chooses the appropriate slice and the vessel positions are copied to the upper and lower slices. Based on this information, the position and angulation of the labeling focus is then optimized for each slice. The flow-chart to perform the routine is presented in figure 1 and schematically visualized in figure 2. This information about the positions and angulations of the labeling foci is subsequently transformed to the scanner coordinate system and transferred to the MRI scanner. To evaluate the accuracy of the algorithm, the signal intensities of individual perfusion territories of the ICAs acquired with manual and automatic planning were compared. Prior, the signal intensities of both approaches were normalized to a non-selective ASL scan. All ASL measurements were acquired with the same set of parameters: pCASL tagging, 3D single-shot GraSE read-out with a resolution of 2.75x2.75x5mm³ and 1800ms label duration and 2000ms post labeling delay prior image acquisition. For selective tagging gradient moments of 1.08 mT/m of the additional transversal gradients were used [1]. The measurements were performed on a Philips Achieva 3T scanner (Philips Healthcare, Best, The Netherlands) and included three healthy volunteers.Results and Discussion
The results after applying the Hough transform and the subsequent optimizations is visualized in figure 2. The algorithm was tested on identifying major arteries in the neck. The normalized (to the non-selective acquisition) results of the volunteers are shown in the table in figure 3. These first results show that the performance of the presented algorithm is not substantially different to manual planning of an experienced user, making it a feasible option, especially for less experienced users. For applications distal the Circle of Willis, the approach needs to be refined in order to detect the right amount of smaller arteries as well as taking the distance to other arteries into account to avoid labeling of multiple arteries. Processing time (excluding TOF image data export from the scanner) was 40 seconds including manually choosing the optimum middle slice for tagging.Conclusion
Using the presented approach it is possible to automate the positioning of the labeling focus in super-selective ASL with minimal user interaction when the major brain-feeding arteries are of interest.Acknowledgements
No acknowledgement found.References
[1] Helle M et. al. Magn Reson Med 2010;64:777-86
[2] Illingworth J et. al. IEEE TPAMI 1987;5:690-98
Figures
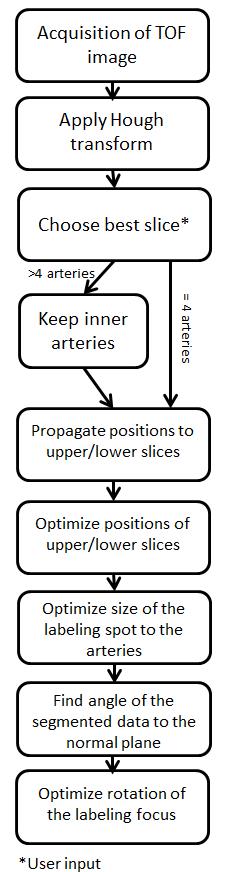
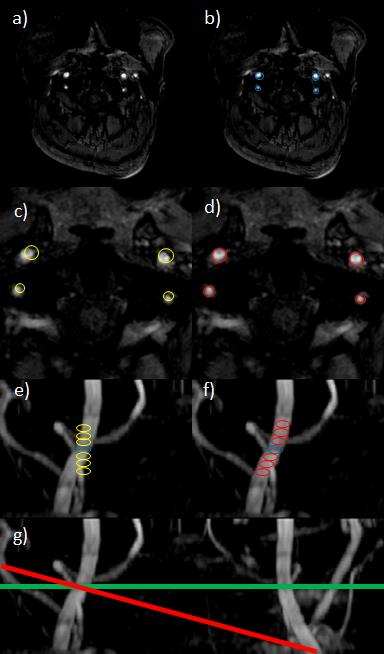

Figure 3: Table: Comparison of the signal of manual and automatic planning normalized to the non-selective acquisition. Selective imaging is generally lower in signal than non-selective acquisitions, which can be explained due to the lower tagging efficiency [1]. An exception is the right ICA of the third volunteer. This might be explained due to optimizing the labeling focus to the individually tagged vessel. Image: Direct comparison of manual (left) and automatic planning (right) after quantification of CBF values.