3805
A 3D printed perfusion phantom for quality controlled measurement of arterial spin labeled perfusion1Bioengineering, UT Dallas, Richardson, TX, United States, 2Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 3Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
Arterial spin labeling (ASL) is a rapidly growing area of interest, primarily because of its ability to provide non-contrast quantitative perfusion maps. For the technique to be adopted for clinical use, these quantitative measurements need to be accurate and robust, which will require a quality controlled perfusion phantom to ensure consistency for different magnet strengths and manufacturers. In this study, we demonstrate a 3D printed perfusion flow phantom that can be easily replicated, and used to test the precision and repeatability of ASL perfusion measurements.
Introduction
Arterial spin labeling (ASL) is a non-contrast perfusion imaging technique that has clinical value because it can easily provide quantitative perfusion maps. This could be beneficial in measuring therapeutic response in various diseases, however, for ASL perfusion imaging to be widely adopted for clinical use, it is important to demonstrate that the technique provides accurate and reliable quantitative perfusion measurements. In this study, we developed a 3D printed perfusion phantom that can be used as a quality control (QC) phantom to evaluate the precision and repeatability of arterial spin labeled perfusion measurements.Methods
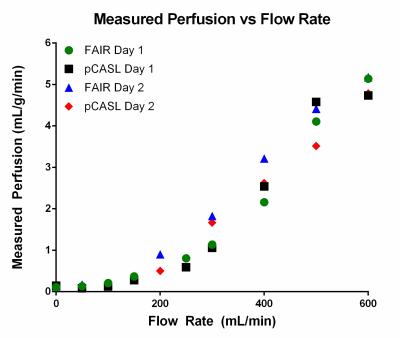
The perfusion phantom was designed to split inflowing water into multiple branches, mimicking the branching of arterial vessels (Figure 1). The branches terminate at small evenly-spaced holes leading into the large chamber that can be fitted with tissue-mimicking material, such as sponge. The small evenly-spaced holes ensure that the entire sponge is completely perfused. The water then leaves the phantom through a mirror image of the input branches. The phantom was submerged in a water bath to minimize B0 inhomogeneities. A pump outside the MR scanner room circulated water through the tubing, and was set to different flow rates to create varied perfusion effects in the sponge. Perfusion was measured with 2D FAIR and 2D pCASL using a single shot turbo spin echo (SShTSE) acquisition. Quantitative perfusion values were calculated using the standard ASL models for each technique.1,2 pCASL labeling was applied axially over the inflow tube for 6 seconds, followed by a 500ms delay before imaging. The inversion time for FAIR was 3 seconds. The pump flow rate was varied from 0 to 600 mL/min to vary perfusion in the sponge (Figure 3). A proton density weighted image was also acquired through the sponge at each flow rate for perfusion quantification. ASL measurements were repeated using the same pump flow rates on two different days to evaluate the repeatability of ASL measured quantitative perfusion. Finally, the agreement between FAIR and pCASL quantitative measurements was assessed at each flow rate.Results
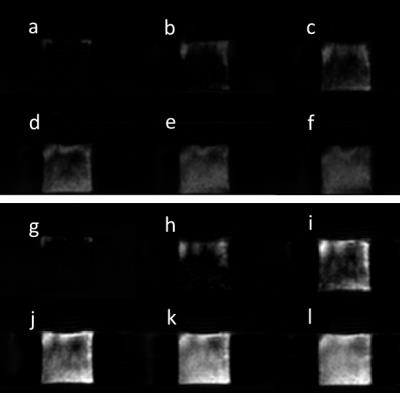
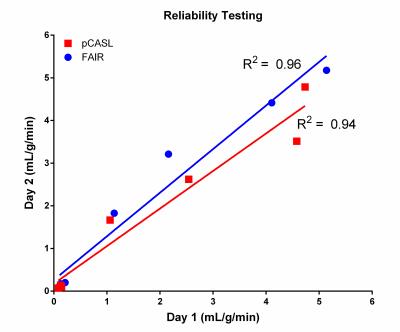
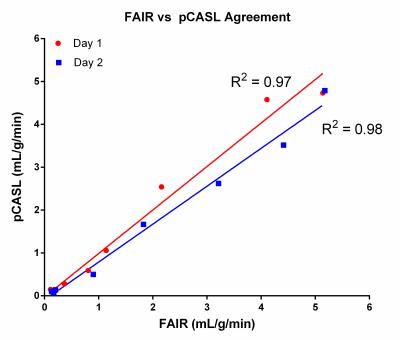
Figure 2 shows axial perfusion weighted images acquired with FAIR and pCASL at increasing pump flow rates. The improved SNR offered by the continuous labeling technique, pCASL (fig. 2, g-l), over pulsed-ASL technique, FAIR (fig 2, a-f), is apparent in these perfusion images. Figure 3 shows the increasing measured perfusion as the inflow rate increases for both ASL techniques on two different days. The delay in the observed increase in perfusion from 0-200 mL/min can likely be attributed to the later arrival of the labeled perfusion bolus, and could potentially be corrected by using a shorter post-labeling delay. Figure 4 demonstrates highly repeatable, quantitative measurements between two scan dates, and figure 5 shows that FAIR and pCASL provide the same perfusion measurements, despite the significant differences in SNR seen in figure 2.Discussion
We demonstrated a simple 3D printed perfusion phantom that can serve as a quality control to ensure that ASL perfusion measurements are reliable and precise, and show that various ASL techniques can provide the same quantitative values. Additionally, this 3D printed phantom can be easily replicated to be used at different sites to compare the measurements made by scanners from different manufacturers and at different field strengths. Future experiments with this phantom will include varying the label duration and post labeling delays to ensure that measured perfusion agrees with the quantitative perfusion model, and defining an inflow range in which this phantom design can provide an accurate linear increase in measured perfusion.Acknowledgements
No acknowledgement found.References
1. Buxton, Richard B., et al. "A general kinetic model for quantitative perfusion imaging with arterial spin labeling." Magnetic resonance in medicine 40.3 (1998): 383-396.
2. Robson, Philip M., et al. "Strategies for reducing respiratory motion artifacts in renal perfusion imaging with arterial spin labeling." Magnetic Resonance in Medicine 61.6 (2009): 1374-1387.
Figures