3799
Mulitplexed OxFlow for Rapid Estimation of Whole-Brain Oxygen Consumption at Rest and During Apneic Challenge1Radiology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
The OxFlow method provides a global CMRO2 estimation in a single pulse sequenc by combining phase-contrast-based flow mapping with estimation of venous oxygen saturation via susceptometry-based oximetry. However, four-interleave in convenional OxFlow makes it difficult to measure rapid changes of brain metabolic states such as response to apneic stimuli. Thus, in this work a highly efficient, multiplexed imaging based OxFlow method was developed and its feasibility was demonstrated in estimation of dynamic CMRO2 in response to an apneic challenge.
Background and Purpose
The cerebral metabolic rate of oxygen (CMRO2), which reflects metabolic activity of the brain at rest and under pathophysiologic challenges, can be estimated via Fick’s principle as CMRO2 = Ca·CBF·(Ya-Yv) where Ca is the blood oxygen carrying capacity, CBF is the cerebral blood flow, and Ya and Yv are the hemoglobin oxygen saturations of arterial and venous blood, respectively. The OxFlow method1 provides a global CMRO2 estimation in a single pulse sequence, in which phase-contrast MRI for neck flow quantification is interleaved with spoiled gradient-echo imaging with two different echo times (TE) at a slice location in the brain for estimation of Yv in the superior sagittal sinus (SSS) via susceptometry-based oximetry1,2, and has been used to evaluate the effect of hypercapnia on CMRO2. However, the four interleaves in the sequence make it difficult to measure rapid changes of brain metabolic states such as response to apneic stimuli. Although a single-slice-based OxFlow method4 has been shown to be able to capture the effect of short stimuli, its underlying assumption of a constant ratio of SSSBF to tCBF may not hold true for patients with hemodynamic malfunctions. Thus, the purpose of this work was to develop a highly efficient, multiplexed imaging based OxFlow method, and demonstrate its feasibility in estimating dynamic CMRO2 under an apneic challenge. Two different types of multiplexed OxFlow were investigated: 1) simultaneous-echo-refocusing (SER)5-based OxFlow and 2) simultaneous-multi-slice (SMS)6-based dual-band OxFlow.Methods
SER OxFlow: A timing diagram of SER OxFlow is shown in Fig. 1a. A pair of RF pulses are successively applied to brain and neck slices. During the brief inter-pulse interval, Tprep, a preparation gradient (Gprep) is inserted to refocus the magnetization in the brain slice after that in the neck slice. For sufficient separation of the two slice signals, a de-phasing gradient (Gd) is located in between the two signal encodings. The zeroth moment of Gprep (A) is set as A=B+C+D where C is the zeroth moment of Gd (Fig. 1a). Given the configuration, the SER OxFlow method achieves the imaging efficiency by having phase-encoding, flow sensitizing, and spoiler gradient pulses shared between the two slices.
Dual-Band OxFlow: Figure 1b shows the sequence configuration of dual-band OxFlow, wherein a dual-band RF pulse is applied to simultaneously excite both neck and brain slices while its phase is cycled by π over the entire pulse train7. Compared to conventional OxFlow, the number of interleaves is reduced from four, resulting in two-fold scan-time shortening. Slice separation was performed by solving the SENSE equation8. Prior to dual-band imaging, single-band data were acquired for coil sensitivity calibration.
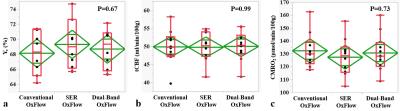
Baseline CMRO2 Estimation: To evaluate the performance of the multiplexed OxFlow methods, data was acquired in ten healthy subjects at 3 T (Siemens Tim Trio) using conventional, SER, and dual-band OxFlow with 16-channel head/neck coils. Common imaging parameters: FOV = 208 mm2, slice thickness = 5 mm, matrix = 2082, bandwidth/pixel = 481 Hz, VENC = 60 cm/s, TR = 30 ms, ΔTE = 3.71 ms. Parameters specific to conventional OxFlow: flip angle = 22°, scan time = 25 sec. Parameters specific to new methods: flip angle = 16°, scan time = 12.5 sec. Derived quantities Yv, tCBF, and CMRO2, from the three methods were statistically compared by ANOVA.
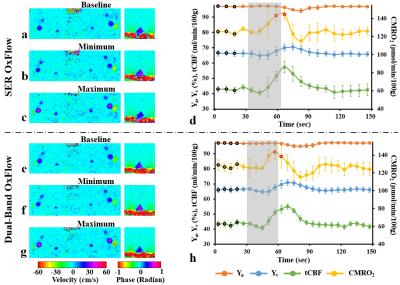
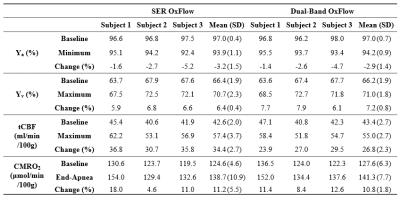
Dynamic CMRO2 Estimation Under Apneic Challenge: The feasibility of the proposed methods for time-resolved CMRO2 estimation was examined at 3 T (Siemens Prisma) in three male subjects using 20-channel head/neck coils. All imaging parameters were kept identical to those above, but TR was adjusted in each method to yield a temporal resolution of 8 (SER) and 6 (dual-band) seconds with flip angles of 12° and 10°, respectively. Subjects were instructed to voluntarily go through three successive stages of baseline, apnea, and recovery through visual and auditory guidance. Ya was continuously monitored by pulse oximetry.
Results
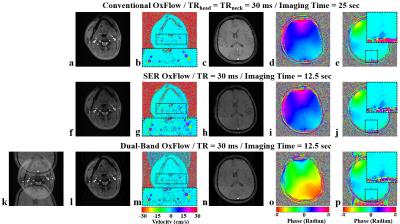
Figure 2 shows representative images acquired with the three methods (see figure legend for details). Scatter plots of Yv, tCBF, and CMRO2 for ten subjects in Fig. 3 suggest the measured parameters not to be significantly different among the three techniques. Fig. 4 highlights the expected functional contrast at baseline, during, and after stimulation for tCBF and Yv, along with the plotted time-courses of all four physiologic quantities clearly reflecting the temporal alterations in response to the apneic challenge. The corresponding estimates in each subject are summarized in Table 1.Conclusion
The results suggest that both multiplexed OxFlow techniques are suited for dynamic estimation of global CMRO2 during short-time stimulation.Acknowledgements
This work was supported by the NIH grant RO1-HL122754.References
1. Jain V, Langham MC, Wehrli FW. MRI estimation of global brain oxygen consumption rate. J Cereb Blood Flow Metab. 2010;30:1598-1607.
2. Fernandez-Seara MA, Techawiboonwong A, Detre JA, Wehrli FW. MR Susceptometry for measuring global brain oxygen extraction. Magn Reson Med. 2006;55:967-973.
3. Jain V, Langham MC, Floyd TF, Jain G, Magland JF, Wehrli FW. Rapid magnetic resonance measurement of global cerebral metabolic rate of oxygen consumption in humans during rest and hypercapnia. J Cereb Blood Flow Metab. 2011;31:1504-1512.
4. Rodgers ZB, Jain V, Englund EK, Langham MC, Wehrli FW. High temporal resolution MRI quantification of global cerebral metabolic rate of oxygen consumption in response to apneic challenge. J Cereb Blood Flow Metab. 2013;33:1514-1522.
5. Feinberg DA, Reese TG, Wedeen VJ. Simultaneous echo refocusing in EPI. Magn Reson Med. 2002;48:1-5.
6. Larkman DJ, Hajnal JV, Herlihy AH, Coutts GA, Young IR, Ehnholm G. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J Magn Reson Imaging. 2001;13;313-317.
7. Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled Aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med. 2005;53;684-691.
8. Pruessman KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;22:952-962.
Figures