3790
Fast Field-Cycling NMR of human glioma resections: characterization of heterogeneity1University of Aberdeen, Aberdeen, United Kingdom, 2U1205 BrainTech Lab, INSERM, Grenoble, France, 3INAC-SCIB, CEA, Grenoble, France
Synopsis
FFC-NMR is a unique tool for the measurements of molecular dynamics in the range of nano- to microseconds. With the development of FFC-MRI scanner, it is now possible to investigate new contrasts using field-dependant variations of T1 to obtain quantitative markers in diseases. In this work we investigate what information FFC-NMR can provide in the context of glioma, using frozen human brain resection from glioma and epileptic surgery. We found that the quadrupolar peaks and T1 dispersions values may be useful biomarkers.
Purpose
Fast- Field-cycling NMR (FFC-NMR), is an NMR method that allows measuring the variations of the longitudinal relaxation time T1 over a wide range of magnetic fields, typically from 1 T down to earth field or even below. Contrary to standard NMR, which operates at strong and fixed magnetic field, FFC-NMR allows switching main magnetic field quickly compared to T1. This provides T1 NMR dispersion profiles (NMRD profiles) that relate quantitatively to molecular dynamics providing valuable structural information over a wide scale of motion length, from nanometers to hundreds of micrometers. It also shows Quadrupolar Peaks (QP) due to proton-nitrogen coupling that are invisible to conventional NMR. Several works demonstrated the usefulness of the T1 NMRD profiles to characterize biological tissues 1,2 and diseases 3,4 by exploiting either the T1 dispersion profiles or the QP. In this work we present the first results of FFC-NMR measurements on a variety of human glioma obtained from surgery. Our goal is to highlight the advantages of FFC-NMR over existing diagnostic techniques and specifically to show if FFC-NMR provides pertinent and complementary information on glioma disease mechanisms, which are still challenging to characterize non-invasively.Subjects and Methods
5 samples of human brain glioma and 3 reference samples of human epileptic brain were obtained frozen from a tissue bank (Grenoble centre for biological resources). Histological analyses were performed to target homogeneous regions. The target regions were cut while frozen. FFC-NMR acquisitions were performed at 37 oC using a SpinMaster relaxometer (Stelar, s.r.l., Italy) using an inversion recovery CPMG sequence using Fomblin (Sigma-Aldrich) to prevent sample drying. The T1 dispersion profiles were acquired from 10 mT to 1 T and fitted using standard models obtained from the literature 5,6 . Mass spectroscopy (MALDI-MS) was also acquired to define the range of tumor protein mass.Results
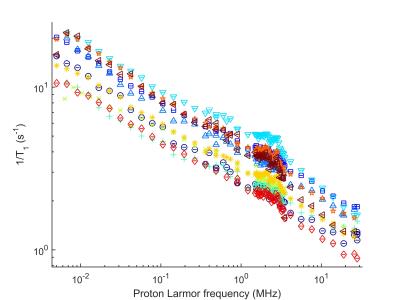
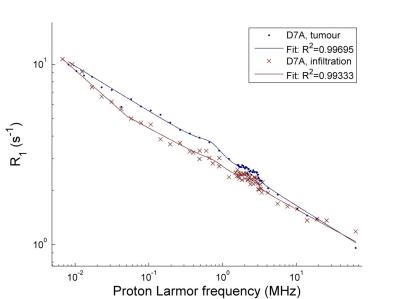
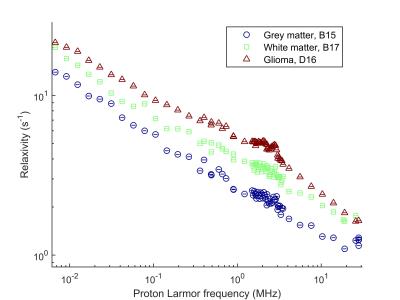
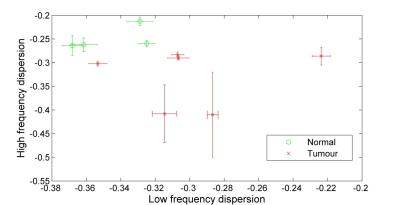
The tumour dispersion curves showed power-law shapes, suggesting relaxation by protein matrix. The low-frequency region showed large differences between glioma and epileptic tissues, correlating with the mass distribution provided by MALDI-MS, possibly indicating variations in protein interactions. Altered tissues microstructures showed large QPs and distinct shape with higher overall relaxation rate (Figure 1). One glioma sample presented regions of infiltrated brain tissues and others of solid tumors, which allowed comparing the NMRD profiles: we observed a very significant change in the slope of the dispersion at low field, indicating differences at slow motions. This may be used to characterize the peritumoral region against the tumor (Figure 2). Grey and white matter did not show large differences in the shape of the NMRD profile, and also exhibited relatively small QP (0.3 to 1 s-1), whereas tumors showed QP amplitudes varying over a larger range (0.3 to 2 s-1) (Figure 3). The slope of the NMRD profiles at low and high fields also showed much variations compared to the error, indicating a possible source of contrast (Figure 4).Discussion and conclusions
Contrast between tumour grades was more pronounced at low magnetic field for several samples, such as infiltrated tumour against solid tumour, and QP appeared as a potential biomarker of tissue remodelling. This work also showed significant differences between epileptic tissues, here considered as a standard reference and glioma that can be exploited for tissue structure characterisation. This pilot work will be extended to better understand the correlations between tissue structures and the T1 dispersion curve and highlights the potential of FFC-NMR to provide novel relevant contrasts.Acknowledgements
No acknowledgement found.References
1. Koenig SH & Brown III RD, Prog. Nucl. Magn. Reson. Spectrosc. (1990).
2. Jiao X & Bryant RG, MRM. (1996).
3. Broche LM et al. MRM. (2012).
4. Lurie DJ et al. Comptes Rendus Phys. (2010).
5. Sunde EP & Halle B, JMR. (2010).
6. Kimmich R. & Anoardo E, Prog. Nucl. Magn. Reson. Spectrosc. (2004).
Figures