3788
High Resolution Magic Angle Spinning (HR-MAS) NMR characterization of inhomogeneous magnetization transfer (ihMT) responsive samples1Aix Marseille Univ, CNRS, CRMBM, Marseille, France, 2Aix Marseille Univ, CNRS, ICR, Marseille, France, 3Division of MR Research, Beth Israel Deaconess Medical Center, Harvard Medical School
Synopsis
Inhomogeneous magnetization transfer (ihMT) is a promising technique for central nervous system imaging. Although ihMT signal is mostly observed in myelinated tissues, weaker ihMT signal may be revealed in other biological tissue. The fundamental relationship between the underlying NMR lineshape and the ihMT signal is still an open question. Here we investigate high resolution magic-angle spinning (HR-MAS) NMR to provide insight into the mechanisms underpinning line broadening of ihMT responsive samples. The resulting spectra evidence different dipolar Hamiltonian components contributing to line broadening and support ihMT as being dominated by inhomogeneous interaction in myelinated tissues.
Introduction
Inhomogeneous magnetization transfer (ihMT) is a promising technique for imaging myelinated tissues (1,2). The ihMT signal arises from the dipolar broadening of macromolecular NMR lines and has been shown to be related to the relaxation time of the corresponding dipolar order T1D (3). Although ihMT signal is mostly observed in relatively long T1D myelinated tissues at RF powers available in clinical scanners, weaker ihMT signal may be revealed in other biological tissue or compounds exhibiting short T1Ds, such as muscle or agarose (4). Whereas NMR thermodynamic models (spin bath) provide a satisfying description of the observe ihMT signal (3), in relation to T1D, the fundamental relationship between the underlying NMR lineshape and the ihMT signal is still an open question. In the current study, high resolution magic-angle spinning (HR-MAS) NMR is used to further characterize ihMT responsive sample lineshapes to provide insight into the mechanisms underpinning line broadening.Materials and Methods
Samples: Rat Spinal Cord (SC) and muscle specimens were obtained from wistar strain. SC and muscle samples were prepared to fit into the small HR-MAS probe. These samples were soaked in D2O for 24h to reduce the free water signal. In addition to SC and muscle, samples of 4% agarose gel prepared in D2O and wood (Mediterranean Pine) were also studied.
Magic-angle spinning: Experiments were performed on a Bruker 9.4 T magnet operated by an AVANCE III HD NMR spectrometer and equipped with a Bruker 4-mm HR-MAS probe. The sample temperatures were adjusted from 20°C to 37°C using a gas flow cryostat, and the values reported were monitored using an external reference. The 1H NMR experiments were recorded using 5 ms 90° pulse duration and spinning frequencies ranging from 1 kHz to 4 kHz.
Results
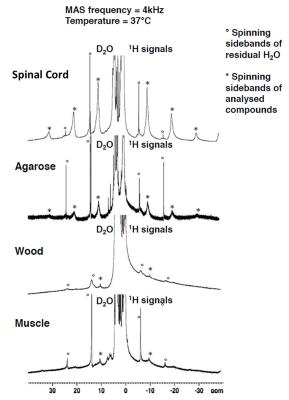
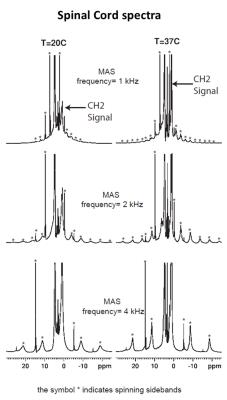
The comparison between the experimental 1H MAS spectra obtained from the various samples evidence different HR-MAS averaging effects underlying line broadening mechanisms (Fig.1). In the case of SC, the methylene proton signal, arising from the lipid chains, gives rise to intense and sharp spinning side bands. In contrast, the methylene proton signal of muscle gives rise to weaker, broad and fewer detectable sidebands. Spinning sidebands are evidenced in agarose as well but hardly distinguishable in wood. Fig. 2 shows the HR-MAS spectra for SC as a function of spinning frequency and temperature. Spinning sidebands are distinguishable from the slowest 1 kHz spinning frequency (Fig.2) and are more pronounced at 37°C as compared to 20°C.Discussion
HR-MAS is usually used for biological tissue studies to remove dipolar broadening by rotational averaging and to reveal the underlying metabolite signal carrying previous information. The residual line broadening observed in the HR-MAS 1H spectra, for a given spinning rate, depends on the nature of the dipolar couplings (5): inhomogeneous interactions are removed at low spinning rate and give rise to sharp spinning sidebands, whereas a purely homogeneous interaction requires spinning rate faster than the static linewidth for efficient averaging. Then, in essence the presence of spinning sidebands at low spinning rate is a signature of an inhomogeneous component in the 1H-1H dipolar Hamiltonian. Within this framework the differences observed between SC and muscle spectra (sharper sidebands for SC evidenced at slower spinning frequencies) indicate different dipolar Hamiltonian components. This is presumably attributed to a strong inhomogeneous component of the myelin-lipid spectrum (6) and more efficient homogenization processes occurring in muscle tissue, leading to faster spectral diffusion. Our study has been conducted for different temperatures and demonstrates that for SC (Fig. 2), at temperatures around 37°C, spinning sideband intensities increase and become sharper. Conversely for muscle, agarose and wood, spinning sideband intensities remain weak. These observations support ihMT as being dominated by inhomogeneous interaction in myelinated tissues (although purely homogeneous interaction may, in principle, give rise to ihMT signal (7,8)) and are consistent with longer T1D and higher ihMT ratio observed in SC at 40°C as compared to 20°C (9) and compared to other samples (4). T1D may be seen as a time constant driven by spin diffusion (flip-flop) which defines a time scale for line homogenization.Conclusion
IhMT responsive tissue specimens and samples have been characterized by HR-MAS and revealed distinct line averaging features with more pronounced sharper spinning sidebands in SC tissue. This evidences different dipolar Hamiltonian components contributing to line broadening and is presumably related to longer T1D and stronger ihMT signal measured in myelinated tissues. On the basis of these results, we will explore the theoretical relationship between the NMR lineshapes and ihMT.Acknowledgements
No acknowledgement found.References
1. Varma G, Duhamel G, de Bazelaire C, Alsop DC. Magnetization Transfer from Inhomogeneously Broadened Lines: A Potential Marker for Myelin. Magn. Reson. Med. 2015;73:614–622.
2. Girard OM, Prevost VH, Varma G, Cozzone PJ, Alsop DC, Duhamel G. Magnetization transfer from inhomogeneously broadened lines (ihMT): Experimental optimization of saturation parameters for human brain imaging at 1.5 Tesla. Magn. Reson. Med. 2015;73:2111–2121.
3. Varma G, Girard OM,
Prevost VH, Grant AK, Duhamel G, Alsop DC. Interpretation of magnetization
transfer from inhomogeneously broadened lines (ihMT) in tissues as a dipolar
order effect within motion restricted molecules. J. Magn. Reson. 2015;260:67–76.
4. Duhamel G, Prevost VH, Varma G, Alsop DC, Girard OM. T1D-W IhMT: Dipolar Relaxation Time Weighted Imaging Using Inhomogeneous Magnetization Transfer. In: Proceedings of the ISMRM. Singapore; 2016. p. 2909.
5. Chen J-H, Singer S. High-Resolution Magic Angle Spinning NMR Spectroscopy. In: The Handbook of Metabonomics and Metabolomics. Elsevier. 2007.
6. Chen J-H, Sambol EB, Decarolis P, O’Connor R, Geha RC, Wu YV, Singer S. High-resolution MAS NMR spectroscopy detection of the spin magnetization exchange by cross-relaxation and chemical exchange in intact cell lines and human tissue specimens. Magn. Reson. Med. 2006;55:1246–1256.
7. Korb J-P, Maruani J. Pulse saturation behavior of inhomogeneously broadened lines. I. Slow spectral diffusion case. Phys. Rev. B 1981;23:971–975.
8. Manning AP, Chang KL, MacKay A, Michal CA. IHMT: Is it misnamed? A simple theoretical description of “inhomogeneous” MT. In: Proceedings of the ISMRM. Singapore; 2016. p. 305.
9. Swanson SD, Malyarenko DI, Fabiilli ML, Welsh RC, Nielsen J-F, Srinivasan A. Molecular, dynamic, and structural origin of inhomogeneous magnetization transfer in lipid membranes. Magn. Reson. Med. 2016, ePub