3774
Dual-CEST: a novel 3D-CEST sequence exploiting simultaneous transverse and longitudinal CEST signal encoding.1Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2Department of Engineering Science, University of Oxford, Oxford, United Kingdom
Synopsis
We propose a novel 3D method called Dual-CEST, which simultaneously captures the CEST contrast stored in the longitudinal and transverse magnetisation of the bulk water. By including ADC events and a hexagonal spoiling scheme during the CEST preparation phase we collected the usually neglected transverse signal, generated by direct water saturation (a method we call NoXi-CEST). The unaffected longitudinal magnetisation is then acquired using a 3D-GRASE readout resulting in one (Dual-CEST) sequence (3:15 min) that provides XY-spectra, in addition to conventional Z-spectra, and producing additional CEST-contrast and B0-maps without loss of contrast or additional scan time (compared with conventional imaging).
Background & Purpose
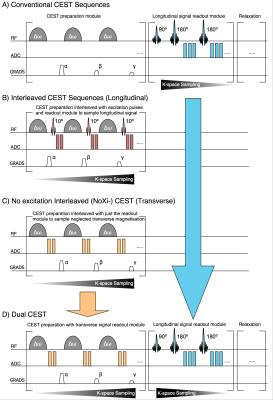
Conventional CEST sequences (Fig. 1A), with preparation and readout separated, have high CNR and SNR, but RF duty-cycle requirements make them time-inefficient. Interleaved 3D-CEST imaging was proposed to address this issue (Fig. 1B).1 However, the additional excitation pulses used for signal readout by these sequences lower the achievable steady state longitudinal magnetisation, and hence the CEST contrast that can be established. This is especially problematic in-vivo where this contrast loss is large compared to the encoded CEST contrast.2
Recently, experiments with SSFP-CEST have shown that CEST contrast can be obtained with interleaved sequences, but without additional excitation pulses, by utilising the transverse signal generated by multiple CEST-preparation pulses.3 Short TR and balanced gradients used in these methods make it difficult, however, to separate the different contributions to the signal. The short-duration RF pulses also make selective excitation of CEST agents problematic. Lastly, direct excitation of the water, and with it creation of transverse magnetisation, is reduced at increasing off-resonance frequencies, making it challenging to image CEST agents further from the bulk water frequency (contrary to other methods).
Published CEST methods to date, whether interleaved or not, have focused on deriving contrast from either the longitudinal- or transverse-magnetisation component. Recognising the strengths, weaknesses and complementary nature of both approaches, we sought to design a pulse sequence that combines these two methods simultaneously to create a single sequence that is more efficient than either approach alone.
Methods
In 3D-interleaved CEST, any residual transverse signal generated by direct water saturation from the CEST pulses is spoiled, after which a readout pulse at the water frequency samples contrast from the longitudinal magnetisation (Fig. 1B). By removing the readout pulses and switching the spoiler and ADC events, a sequence that acquires just this residual transverse signal is obtained, which forms the first half of Dual-CEST (Fig. 1C). We name this approach ‘no Excitation Interleaved CEST’ (NoXi-CEST).
This method differs from SSFP in two ways: firstly the TR of 40ms is far from the standard SSFP domain (TR<<T1,T2), making sequence timings compatible with conventional methods; secondly, an unbalanced cycled hexagonal gradient spoiling scheme is used.2,4 Both strategies deliberately isolate the contribution of a single CEST pulse, thus avoiding contributions from previous TRs.
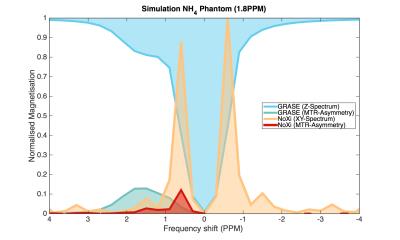
Conventional methods use constant flip angles for readout (Fig. 1B). NoXi-CEST captures the signal that is lost with direct water saturation, a symmetrical function of off-resonance frequency, resulting in varying readout signal. The resulting XY-spectrum is thus a convolution of the Z-spectrum with this function. Simulation of both spectra was done in Matlab (Fig. 2).
As the ADC events used in NoXi-CEST are placed before spoiling gradients and have no influence on the longitudinal magnetisation, the Z-spectrum can be acquired simultaneously by adding a 3D-GRASE readout to NoXi-CEST. Capturing both parts of the signal, we name this combination Dual-CEST (Fig. 1D). Conversely the ADC events for the GRASE readout occur after NoXi-CEST, thus both parts of Dual-CEST can be considered separately, allowing for individual customisation of the imaging parameters.
Dual-CEST does not prolong sequence acquisition (in our case 3:15min) over conventional CEST, as the identical preparation can be shared and the additional ADC events of NoXi-CEST occur during the dead time dictated by the duty cycle of clinical systems.
A compartmental ammonium chloride phantom [0-1M] (fixed T1=1000ms) was scanned at 3T (Siemens Verio). Both XY- and Z-spectra were normalised and B0-corrected using the WASSR5 method after which MTR-asymmetry was calculated to generate CEST contrast maps in Matlab.
Results and Discussion
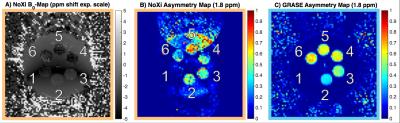
As predicted by simulations (Fig. 2), NoXi-CEST generates high signal near the resonance of the bulk-water from which high quality B0-maps were also obtained (Fig. 3A).
Even though NoXi-CEST has low SNR for points far from the bulk water (Fig. 2), making artefacts there more pronounced, a significant amount of CEST contrast (Fig. 3B) can be acquired from this normally neglected signal without the need for signal separation. Figure 3C demonstrates the longitudinal CEST contrast acquired from the 3D-GRASE readout after identical preparation.
Dual-CEST, acquiring both spectra, provides additional information to conventional Z-spectra imaging, without any penalty in contrast or acquisition time, and may be used to improve fitting of the individual components contributing to the CEST contrast.
Conclusion
Dual-CEST offers a practical solution to existing acquisition problems by using the complementary strengths of interleaved transverse- and non-interleaved longitudinal CEST imaging. It can be used to image CEST agents close to the water resonance and generates additional CEST contrast, B0-maps and spectral information without any penalty in scan time or contrast compared to conventional methods.Acknowledgements
This work was funded by the EPSRC, Scatcherd European Scholarships and the Dunhill Medical Trust.References
[1] C.K. Jones, D. Polders, J. Hua, H. Zhu, H.J. Hoogduin, J. Zhou, et al., In vivo three-dimensional whole-brain pulsed steady-state chemical exchange saturation transfer at 7 T., Magn. Reson. Med. 67 (2012) 1579–89. doi:10.1002/mrm.23141.
[2] R. Brand, N. Blockley, M. Chappell, P. Jezzard, Clinically Relevant Rapid 3D CEST Imaging with Hexagonal Spoiling Gradients, Optimised B1, and Symmetric Z-Spectrum Sampling, in: 24th Annu. Meet. ISMRM, Singapore, Singapore, 2016: p. 0299.
[3] S. Zhang, Z. Liu, R.E. Lenkinski, E. Vinogradov, Balanced Steady State Free Precession (bSSFP) from an effective field perspective: application to the detection of exchange (bSSFPX), in: 23th Annu. Meet. ISMRM, Canada, Toronto, n.d.: p. 0785.
[4] A.T. Hess, M.D. Robson, Hexagonal gradient scheme with RF spoiling improves spoiling performance for high-flip-angle fast gradient echo imaging., Magn. Reson. Med. (2016). doi:10.1002/mrm.26213.
[5] M. Kim, J. Gillen, B.A. Landman, J. Zhou, P.C.M. van Zijl, Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments, Magn. Reson. Med. 61 (2009) 1441–1450. doi:10.1002/mrm.21873.
Figures