3772
Chemical Exchange Saturation Transfer (CEST) with a Multiple Gradient Echo Chemical Shift Imaging (CSI) Sequence1Department of Imaging Physics, The University of Texas M.D. Anderson Cancer Center, Houston, TX, United States, 2Department of Inverventional Radiology, The University of Texas M.D. Anderson Cancer Center, Houston, TX, United States
Synopsis
Chemical exchange saturation transfer (CEST) is observed by applying off-resonance saturation pulses at multiple frequencies and measuring the change in water signal. However, if lipid molecules are present, signals from both species contribute to overall voxel signal, confounding spectral measurement. A multiple gradient echo sequence was developed to simultaneously perform chemical shift imaging with CEST to decouple these signals and measure the independent effect of off-resonance saturation transfer to water. 2D spectra were reconstructed, from which individual water-only z-spectra were obtained. In data from a mixed species phantom, amide proton peaks were clearly visible despite the presence of lipid signal.
Introduction
Chemical exchange saturation transfer (CEST) is an imaging approach that relies on the exchange of saturated off resonance protons with on-resonance water. This is observed with the change in water signal after the application of off-resonance saturation pulses at multiple frequencies, which are mapped out in a z-spectrum. However, if lipid molecules are present, signals from both species contribute to overall voxel signal, confounding spectral measurement1. We propose the use of a multiple fast gradient echo (MFGRE) acquisition to simultaneously perform chemical shift imaging (CSI) with CEST to decouple these signals to measure the independent effect of off-resonance saturation transfer to water.Methods
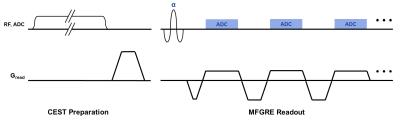
Off-resonance saturation preparation pulses were added to an MFGRE sequence to perform simultaneous CEST and chemical shift imaging (CSI), by collecting 2D data at periodic saturation offset frequencies and gradient echo times. Two sequence acquisition types were implemented: one that applied a saturation pulse to each repetition of the MFGRE sequence with a linear view order2, and another that applied a pulsed saturation preparation for a 5 second duration followed by a single shot MFGRE acquisition with a centric view order3. Rounded rectangular “Fermi” saturation pulses with a duration of 126 msec and peak B1 of 2.0 uT were applied, at frequencies ranging from +7 to -7 ppm at intervals of 0.25 ppm, for a total of 57 offset frequencies. 16 unipolar echoes were acquired for each saturation offset frequency, using two TE-interleaved echo trains for an effective echo spacing of 0.912 msec.
A phantom consisting of four vials immersed in water was imaged in a 3T clinical scanner (MR750W, GE Healthcare, Waukesha, WI). The vials were partially filled with a solution of 120mM Iopamidol in phosphate-buffer saline, and the remainder of each vial was filled with vegetable oil such that the interface between oil and aqueous solutions lay on the same plane. The pH of the aqueous solutions in the vials were adjusted with HCl and NaOH to values of 5, 6, 7, and 8. A single 10mm thick slice at the interface of oil and aqueous solution of all vials was acquired with both sequences to achieve voxels with mixed water and lipid species. Acquisition parameters common to both sequences were: FOV=25.6 cm, matrix=128x128, bandwidth = ±83.33 kHz, ASSET acceleration factor = 2. The linear view order sequence was acquired with TR=139msec, flip=20, and total acquisition time = 22:18, while the centric view order sequence was acquired with TR=21msec, flip = 10, and total acquisition time = 14:58.
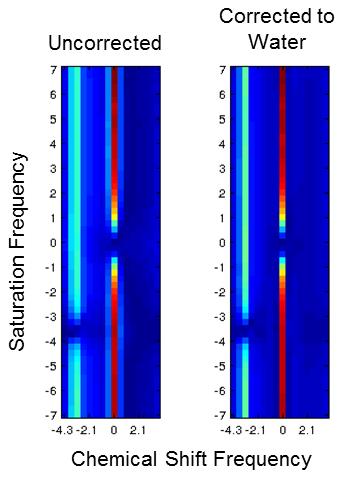
All post-processing was performed with Matlab (Mathworks, Natick, MA). The complex data from all 57 saturation frequency offsets was summed for each echo time, producing a single composite echo train. ARMA modeling4 was performed on this echo train to model the major water and lipid peaks and detect their frequencies. Each echo train could then be centered to the water frequency by multiplying the echo train with a linear phase ramp. After applying this frequency correction to the echo train for each CEST saturation frequency offset, the Fourier transform was performed on each echo train, creating a 2D spectrum of chemical shift and saturation transfer. A water-only z-spectrum was thus obtained by selecting the z-spectrum corresponding to the water frequency.
Results
2D spectra were successfully reconstructed, and water and lipid signals were clearly separated for each saturation frequency offset. Contamination from fat was eliminated in reconstructed water-only z-spectra, which were improved further with correction to the water frequency. Amide proton peaks were clearly distinguished in the water-only z-spectra. Saturation prepared centric readout reduced acquisition time but demonstrated loss of CEST contrast due to the longer repetition times required of a MFGRE readout.Discussion
We have demonstrated simultaneous chemical shift and CEST imaging with a saturation prepared multiple gradient echo sequence. Once water signal is separated from the other species in the voxel, effects of chemical exchange with the water component can be isolated. This may play an important role for assessing CEST effects in fatty tissues such as liver, and aids symmetry analysis of amide proton transfer contrast. CSI inherent in this technique also provides other biomarkers including fat fraction, R2*, and local field shift. Since water frequency is detected in the chemical shift spectrum, B0 correction of the z-spectrum can also be easily derived for improved peak fitting. With sufficient signal, this method may also enable investigation of CEST of other species.Acknowledgements
No acknowledgement found.References
1. Sun PZ, Zhou J, Sun W, Huang J, van Zijl PCM. Suppression of lipid artifacts in amide proton transfer imaging. Magn. Reson. Med. 2005; 54(1): 222–225.
2. Dixon WT, Hancu I, Ratnakar SJ, Sherry AD, Lenkinski RE, Alsop DC. A multislice gradient echo pulse sequence for CEST imaging. Magn Reson Med. 2010; 63(1):253-6.
3. Shah T, Lu L, Dell KM, Pagel MD, Griswold MA, Flask CA. CEST-FISP: a novel technique for rapid chemical exchange saturation transfer MRI at 7 T. Magn Reson Med. 2011; 65(2):432-7.
4. BA Taylor, KP Hwang, JD Hazle, RJ Stafford. Autoregressive moving average modeling for spectral parameter estimation from a multigradient echo chemical shift acquisition. Med Phys. 2009; 36(3):753-64.
Figures