3769
Multi-Band Enhanced Magnetization Transfer Contrast (MBE-MTC) Preparation1Radiology-CMRR, University of Minnesota, Minneapolis, MN, United States, 2Interdisciplinary Institute of Neuroscience and Technology, Zhejiang University, Hangzhou, People's Republic of China, 3Department of Biomedical Engineering, Zhejiang University, Hangzhou, People's Republic of China, 4Siemens Healthcare, Minneapolis, MN, United States
Synopsis
To overcome the limitations of traditional magnetization contrast (MTC) preparation methods, the Multi-Banded (MB) RF-pulse Enhanced Magnetization Transfer Contrast (MBE-MTC) preparation was proposed by using the multi-banded RF pulses for the MTC preparation. Such an approach has been evaluated via studies in the skeleton muscle, kidneys and the brain with demonstrated benefits. The proposed MBE-MTC preparation provides an alternative way for MTI either for increased MTC or simultaneous and symmetric capture of the MTC or its changes in tissue.
Purpose
Magnetization transfer (MT) imaging (MTI) provides a method to investigate the properties of protons bound to macromolecules in tissue (1), and has been applied for diverse clinical applications (2-4). In MTI, off-resonance RF pulses are dominantly used with a bandwidth of several hundred Hz, and applied at a frequency offset at least 1 kHz from the mobile water peak to minimize direct saturation. To achieve off-resonance saturations of bound water spins within a large frequency range, the bandwidth of the MT RF pulses has to be increased, which will require shifting the MT pulses further away from the mobile water to avoid the undesired augmentation of direct saturation. However, shifting the MT pulses further off-resonance decreases the ability to saturate bound spins close to water, and reduces the magnetization transfer contrast (MTC). Furthermore, in a typical MTI, MT pulses are only applied with an offset frequency on one side of the mobile water peak, but the MTC, as well as its changes in tissue, can be asymmetric (5-6). To address the above-mentioned limitations, we propose to use multi-banded RF pulses for MTC preparation, which is referred to as Multi-Banded (MB) RF-pulse Enhanced Magnetization Transfer Contrast (MBE-MTC) preparation. Such an approach has been implemented and demonstrated in healthy volunteers.Methods
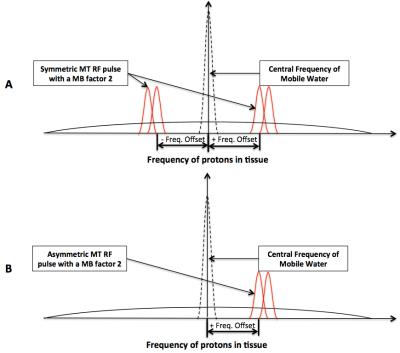
The MBE-MTC preparation utilizes the multi-banded versions of an MT RF pulse for MTC preparation. The MB RF pulses can be applied either symmetrically or asymmetrically with respect to the central frequency of mobile water (Figure 1). Studies were performed with healthy volunteers on a Siemens 3T MRI scanner under an IRB approved protocol with written consent. These studies utilized a Gaussian RF pulse and its multi-banded versions for the MTC preparation, and employed a gradient recalled echo (GRE) sequence as the readout for 3D acquisition in the brain and skeletal muscle, or 2D acquisition of a single slice in the kidneys within a single breath-hold. Studies were first performed in the skeletal muscle, kidneys and the brain to evaluate whether the MBE-MTC preparation can increase the MTC compared to the traditional/standard method. To evaluate the ability of the MBE-MTC approach to detect the MTC asymmetry in the brain, studies were performed with two asymmetric MBE-MTC preparations with an MB factor 2, as well as the traditional method and symmetric MBE-MTC preparation for comparison purposes. For all studies, a 1.2 kHz frequency offset was applied (Figure 1). The body coil was used for RF transmission, a 32-channel phased array receive coil was used for the brain and a combined spine and flexible body coils for other body regions. In the brain study, because of the inhomogeneity of the B0 field, the MTC RF pulses with an offset frequency toward the body direction may introduce potential saturation of inflowing blood, confounding the measurement of MTC asymmetry. Therefore, MTI scans were performed utilizing two 50 mm saturation bands 10 mm below and above the imaging volume.
Post-imaging processing (e.g., co-registration, anatomic image segmentation and magnetization transfer ratio calculation) was performed with the SPM software and scripts in Matlab. Statistical analyses using paired two-tailed t test were performed within the Excel software.
Results and Discussions
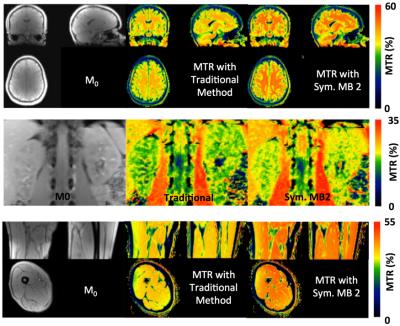
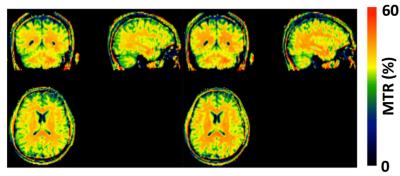
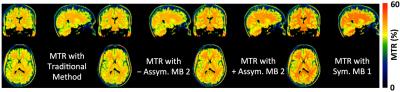
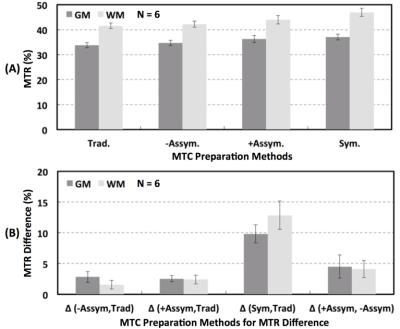
Our initial study results suggest that compared to the traditional method, MBE-MTC preparation using symmetrically positioned RF pulses improved the MTC while staying under the allowed maximum RF power deposition for both methods (Figure 2). The brain study results showed the asymmetry of the MTC regardless of whether the out-of-volume saturation was performed (Figures 3 and 4). The brain studies further indicated that the MBE-MTC preparation provides significantly elevated MTC in comparison to the traditional method, and the magnetization transfer rate (MTR) difference between the positive and negative MBE-MTC preparations is significant. The MTR and MTR percent differences between two different MTC preparations in the brain study are presented in Figure 5.
Our other preliminary data indicates that using larger MB factors can further increase the MTC, which outperforms increasing the bandwidth of the standard Gaussian RF by reducing the RF duration, which requires shifting the RF pulse further away from the water peak to avoid direction saturation (data not shown). The MTR time efficiency as a function of the MB factor is under investigation.
Conclusions
The proposed MBE-MTC preparation provides an alternative way for MTI either for increased MTC or simultaneous and symmetric capture of the MTC or its changes in tissue.Acknowledgements
P41 EB015894, P30 ICC and Human Connectome Project (1U54 MH091657), UMF0003900 and UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.References
1. Wolff, S.D. and R.S. Balaban, Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med, 1989. 10(1): p. 135-44.
2. Rovaris, M., et al., Brain MRI correlates of magnetization transfer imaging metrics in patients with multiple sclerosis. J Neurol Sci, 1999. 166(1): p. 58-63.
3. Kabani, N.J., J.G. Sled, and H. Chertkow, Magnetization transfer ratio in mild cognitive impairment and dementia of Alzheimer's type. Neuroimage, 2002. 15(3): p. 604-10.
4. Yarnykh, V.L., E.V. Tartaglione, and G.N. Ioannou, Fast macromolecular proton fraction mapping of the human liver in vivo for quantitative assessment of hepatic fibrosis. NMR Biomed, 2015. 28(12): p. 1716-25.
5. Kim J, Wu Y, Guo Y, Zheng H, Sun PZ. A review of optimization and quantification techniques for chemical exchange saturation transfer MRI toward sensitive in vivo imaging. Contrast Media Mol Imaging. 2015;10(3):163-178.
6. Hua J, Jones CK, Blakeley J, Smith SA, van Zijl PC, Zhou J. Quantitative description of the asymmetry in magnetization transfer effects around the water resonance in the human brain. Magn Reson Med. 2007 Oct;58(4):786-93.
Figures