3767
Robust image registration in CEST-acquisitions by exploiting a low-rank plus sparse decomposition of the Z-spectrum1Department of Diagnostic and Interventional Radiology, University Hospital of Würzburg, Würzburg, Germany, 2Comprehensive Heart Failure Center, University Hospital of Würzburg, Würzburg, Germany
Synopsis
A robust motion correction technique for in vivo CEST-acquisitions is proposed. The algorithm exploits a low-rank plus sparse decomposition of the Z-spectrum to separate signal variations due to different off-resonance preparations from accompanying motion. The method was validated in a phantom study and subsequently used to register a CrCest acquisition of the human thigh during exercise.
Target audience
Clinicians and MR physicists interested in CEST-MRI.Purpose
Chemical exchange saturation transfer (CEST, [1])
imaging enables unique contrasts for the non-invasive investigation of
physiological processes at the molecular level. This usually demands the
acquisition of a series of images with varying off-resonance preparation,
leading to rather long acquisition times. As any accompanying motion impairs
the analysis of the resulting Z-spectrum, registration of the image series is
necessary for many in vivo acquisitions, such as the $$$\small CrCEST$$$ analysis of
skeletal muscles during exercise or the myocardium.
This deformation towards a
static CEST image series, however, is demanding. Similar to perfusion studies
[2, 3], the constantly changing contrast between the images of a Z-spectrum is
impeding the straightforward registration to a single reference image.
In this
work, we propose a robust motion correction technique for CEST-acquisitions,
that is based on a low-rank plus sparse decomposition of the underlying image
series.
Theory
The essential aspect of the proposed registration technique is the separation of the contrast variation induced by the saturation at different off-resonance frequencies, and accompanying motion of the depicted organ: The image series $$$\bf I$$$ of dimensions $$$ x \times y \times n $$$ ($$$x, y$$$ = spatial dimensions, $$$n$$$ = number of acquired off-resonance frequencies) is rearranged as a Casorati matrix $$$\bf C$$$ of dimensions $$$x \cdot y \times n$$$, where each column represents a vectorized image. Subsequently, a low-rank approximation $$$ \bf L$$$ of $$$ \bf C$$$ is calculated by applying a singular value decomposition and thresholding the typically fast decreasing singular values by a threshold of $$$\tau$$$, as for example in [4]. If $$$\tau$$$ is chosen appropriately, $$$ \bf L$$$ mainly holds the information of the contrast change, while the sparse residuum $$$\bf C-L$$$ encodes the motion of the organ. $$$ \bf L$$$ is finally rearranged towards the initial dimensions ($$$x \times y \times n$$$) and each of the $$$n$$$ images of $$$\bf I$$$ is registered to the corresponding image in $$$\bf L$$$, resulting in a motion corrected series $$$\bf R$$$.To improve the robustness, multiple iterations $$$i$$$ of the described procedure can be performed. In this case, a low-rank approximation $$$\bf L_i$$$ of the registered series of the last iteration $$$\bf R_{i-1}$$$ is determined. $$$\bf I$$$ is then registered to $$$\bf L_i$$$, leading to a refined series $$$\bf R_i$$$.
Methods
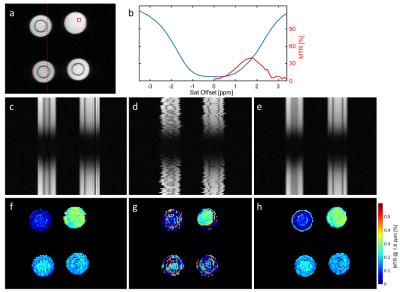
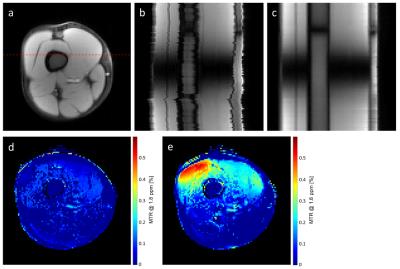
In a first example, agarose phantoms with different concentrations of creatine (30-200mM) were investigated using a typical $$$\small CrCEST$$$ pulse sequence [6] on a 3T MR-system (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). $$$n = 101$$$ images were acquired covering off-resonance ranging from -5ppm to +5ppm (see Fig. 1a,b), and WASSR [7] was used for the correction of B0 field variations. To simulate motion, both CEST and WASSR data were then corrupted by random shifts (mean=0 pixels, SD=1 pixel) and rotations (mean=0$$$^{\circ}$$$, SD=1$$$^{\circ}$$$) in image space, each individually for the different off-resonances (compare Fig. 1c and Fig. 1d). The proposed algorithm was applied to the corrupted data using 10 iterations and a $$$\tau$$$ tuned to 5$$$\%$$$ of the largest singular value, while the method of Myronenko et al. [5] was used to perform the pairwise non-rigid registrations in the last step of each iteration. $$$\small CrCEST_{asym}$$$ maps (@1.8 ppm) were finally determined for the original data, the corrupted data, and the corrupted data registered by the proposed technique.In a second example, the $$$\small CrCEST$$$ analysis described above was performed in a human thigh, both at rest and during exercise. The thigh of the healthy volunteer was stressed via dynamic work during the pauses (10 seconds) between the acquisitions at different off-resonance frequencies. The motion correction was applied (10 iterations, $$$\tau = 2\%$$$ of the largest singular value) to the series acquired under exercise, and $$$\small CrCEST_{asym}$$$ maps were determined for this registered series and the series at rest.
Results
Fig. 1 shows the results of the phantom study. The
spatio-temporal depictions (second row) both show the varying contrast, due to
different off-resonance saturations (up-down), as well as spatial shifts/rotations
throughout the series (left-right). The transformations artificially introduced
within the corrupted dataset (1d) were removed by the proposed procedure
(1e). Only a slight blurring remains in comparison to the original series
(1c). The $$$\small CrCEST_{asym}$$$ maps shown in the third row, illustrate that
already a small jitter of several pixels highly impedes the analysis of the
Z-spectrum (compare 1f and 1g). The great majority of pixels within the map
obtained by analyzing the registered series (1h), however, exhibits
differences to the original values in 1f, that are below the noise level. At
most, the border of the upper left vial shows false values, which is originated
by the already discussed slight drop in spatial resolution.
Similar
results were obtained for the in-vivo example (Fig. 2). The exercise performed
by the volunteer causes a spatial jitter clearly visible in 2b. This is removed
by the application of the proposed image registration (2c), allowing a robust
comparison of $$$\small CrCEST_{asym}$$$ maps at rest and under load (2d and 2e).
Discussion & Conclusion
The proposed algorithm has proven to be robust
for the registration of image series that are typically acquired in CEST
experiments/investigations. This is superior to methods that register each
image of a series to a single reference image, and thereby improves the value
of in-vivo CEST measurements.Acknowledgements
Grant sponsors: Deutsche Forschungsgemeinschaft (DFG KO 2938/4-1), Siemens Healthcare, Comprehensive Heart Failure Center (CF1.8)References
[1] Guivel-Scharen et al., J Magn Reson 133:36-45 (1998) [2] Adluru et al., JMRI 24:1062-1070 (2006) [3] Hamy et al. Med Image Anal 18:301-313 (2014) [4] Otazo et al., MRM 73:1125-1136 (2015) [5] Myronenko et al., IEEE Trans Med Imaging 29:1882-1891 (2010) [6] Kogan et al., JMRI 40:596-602 (2014) [7] Kim et al., MRM 61:1441-1450 (2009)Figures