3765
On the boost effect of inhomogeneous Magnetization Transfer (ihMT)1Aix Marseille Univ, CNRS, CRMBM, UMR 7339, Marseille, France, 2Division of MR Research, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States
Synopsis
A new implementation of inhomogeneous magnetization transfer (ihMT) has recently been introduced, consisting of concentrating the RF energy deposition within the saturation period and demonstrating a significant boost of the ihMT sensitivity. The boost effect has been characterized in this study among different ihMT sequences, species and field strengths and reveals common features. The optimal sequence settings vary with the number of consecutive MT pulses and are presumably related to the timescale of the underlying T1D components and magnetization exchange rates.
Purpose
Inhomogeneous magnetization transfer (ihMT) has been recently presented as a technique sensitive to structures with strong residual dipolar coupling and long dipolar relaxation time T1D, such as myelinated tissues (1). The ihMT signal measured in white matter (WM) has been reported roughly 10% of the unsaturated free water signal. A recent finding demonstrated that significant boost of ihMT sensitivity can be obtained with more efficient use of the energy during the saturation period, by concentrating the power deposition phases, and by interleaved them with exchange periods to allow magnetization exchange from the bound pool to free water (exchange rate R). These novel ihMT implementations have been introduced in human with 3D GRE (2) and 2D prepared SE-EPI sequences (3) at 1.5 and 3T respectively. The current work presents an optimization study of the boost effect on human, completed by a similar preclinical approach on mouse to allow use of stronger saturation intensities (B1sat), limited on human scanners. In addition, for preclinical studies, the boost approach was combined with T1D-filtering (4) allowed by the ihMT technique to study the interdependency of these mechanisms.Method
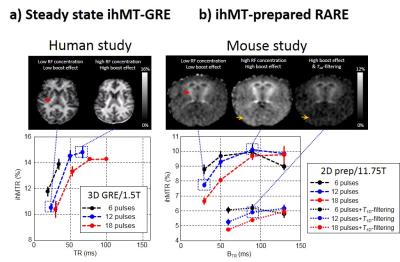
Boosted ihMT techniques: As shown on Figure 1, concentrating the power deposition phases is a self-contained feature in ihMT-GRE approach because of the interleaving of ihMT and readout modules, and is governed by TR. Similarly, a boost sequence design can been implemented on an ihMT prepared 2D-RARE sequence by introducing delays within the presaturation period. The power concentration is in this latter case governed by the Boost Repetition Time (BTR). MR experiments for this study were performed with i) a clinical 1.5T scanner (Avanto, Siemens Healthcare, Erlangen, Germany) on 3 healthy volunteers. 3D ihMT-GRE (TE 4.8ms, 2.5mm iso) sequences were investigated as a function of TR for a constant average power (B1rms = 5.5mT) and bursts of 6, 12 and 18 consecutive MT pulses (pw/Δt=0.5/1ms, Δf=±7kHz (2)). and ii) a Bruker Avance 500 MHz/89mm wide bore vertical imager on anaesthetized healthy mice (10 weeks, n=3). 2D single shot ihMT-RARE (TR/TE=3.4s/1.82ms, 0.3x0.3x1mm) were investigated as a function of BTR for a constant average power (B1rms = 6.7mT) and bursts of 6, 12 and 18 consecutive MT pulses (Δf=±10kHz, pw/Δt=3/3.3ms for strong T1D-filtering conditions, and pw/Δt=1/1.3ms for weak T1D-filtering conditions). Quantitative analyses were performed in highly myelinated pyramidal tracts (PT) for humans and Internal Capsules (IC) for mice.Results
Figure 2 shows ihMTR boost signal evolution as a function of TR and BTR for humans/3D ihMT-GRE and mice/2D ihMT-RARE. In both cases, a similar trend for bell-shaped functions was observed, with first, ihMT signal enhancement with TR/BTR, then reaching a maximum and eventually followed by a decay (clearly visible in mouse at longest BTR values for bursts of 6 and 12 pulses). Figure 2 also suggests that for both humans and mice, bursts of 12 consecutive MT pulses led to optimal boost effect, providing at TR=67ms/BTR=90ms sensitivity gain of ~40-50% compared to that of the most distributed energy deposition scheme (i.e. for shortest TR/BTR values). For T1D-filtered data, the ihMT signal evolution showed similar trend although with lower gain (~5-15%) obtained for the optimal BTR.Discussion
The ihMT boost effect is a common property of steady-state interleaved ihMT-GRE and ihMT prepared single shot readout approaches, with similar trends of the characteristic curves despite differences in species, sequence design, scanners and magnetic field strengths. Of interest, the lower boost effect obtained for the strong T1D-filtering approach compared to that with weak T1D-filtering might indicate that the boost effect is more efficient for short T1D components. This can also be appreciated by the significant ihMT signal increase obtained in muscle (Fig. 2b, orange arrowheads), which has a T1D value of ~2ms, that is 3x lower than WM and Gray Matter(5). The bell-shaped curves describing the ihMT boost effect may indicate that the optimal TR/BTR result from a tradeoff between the exchange rate R and the concentration of RF energy within the ihMT burst. Then, shorter optimized BTR compared to TR obtained in this study would be in agreement with similar concentration of energy ([12 pulses]x[1ms Δt]/[67ms TR]~0.18 for human and [12 pulses]x[1.3ms Δt]/[90ms TR]~0.17 for mouse) and smaller R value for mouse compared to human (1).Conclusion
The boost effect has been characterized among different ihMT sequences, species and field strengths and reveals common features. The optimal sequence settings vary with the number of consecutive MT pulses and are presumably related to the timescale of the underlying T1D components and magnetization exchange rates.Acknowledgements
Support from A*MIDEX grant (n°ANR-11-IDEX-0001-02) funded by the French Government “Investissements d’Avenir” program.The authors thank N.C. for animal handling.References
1. Varma G, Girard OM, Prevost VH, Grant A, Duhamel GD, Alsop DC. Interpretation of magnetization transfer from inhomogeneously broadened lines (ihMT) in tissues as a dipolar order effect within motion restricted molecules. J. Magn. Reson. 2015;260:67–76. doi: 10.1016/j.jmr.2015.08.024.
2. Girard OM, Varma G, MChinda S, Prevost VH, Le Troter A, Rapacchi S, Guye M, Ranjeva J-P, Alsop DC, Duhamel GD. Theoretical and Experimental Optimization of a 3D Steady- State Inhomogeneous Magnetization Transfer (ihMT) Gradient Echo Sequence: Boosting the ihMT Sensitivity with Sparse Energy Deposition. In: International Society for Magnetic Resonance in Medicine. Singapore; 2016.
3. Varma G, Girard OM, Prevost VH, Duhamel G, Alsop DC. Increasing the Inhomogeneous Magnetization Transfer (ihMT) Signal in Vivo with High Amplitude, Low Duty Cycle Irradiation. In: International Society for Magnetic Resonance in Medicine. Singapore; 2016. p. 2890.
4. Duhamel G, Prevost VH, Varma G, Alsop DC, Girard OM. T1D-w ihMT: Dipolar relaxation time weighted imaging using inhomogeneous magnetization transfer. In: International Society for Magnetic Resonance in Medicine. Singapore; 2016. p. 2909.
5. Varma G, Girard OM, Prevost VH, Grant A, Duhamel GD, Alsop DC. In Vivo measurement of a new source of tissue contrast, the dipolar relaxation time, T1D, using a modified ihMT sequence. In: International Society for Magnetic Resonance in Medicine. Singapore; 2016. p. 802.
Figures